Abstract
Objective:
Obesity, particularly child obesity, is one of the most common public health problems in the world and raises the risk of end-stage renal disease. Zinc (Zn) is essential for multiple organs in terms of normal structure and function; however, effects of Zn deficiency or supplementation among young individuals with obesity have not been well studied.
Methods:
Weaned mice were fed high-fat diets (HFD) with varied contents of Zn (Zn deficient, adequate, and supplemented) for 3 or 6 months. This study examined associations between renal pathogenesis and dietary Zn levels, specifically assessing inflammatory pathways by utilizing P38 MAPK inhibitor SB203580.
Results:
HFD feeding induced typical syndromes of obesity-related renal disorders, which worsened by Zn marginal deficiency. The progression of obesity-related renal disorders was delayed by Zn supplementation. HFD induced renal inflammation, reflected by increased P38 MAPK phosphorylation along with increases of inflammatory cytokines MCP-1, IL-1β, IL-6, and TNF-α. P38 MAPK inhibition prevented renal pathological changes in mice fed with HFD and HFD/Zn deficiency.
Conclusions:
P38 MAPK mediated the renal inflammatory responses, which played a central role in the pathogenesis of HFD-induced renal disorders. Zn could delay the progression of obesity-related kidney disease by down-regulating P38 MAPK-mediated inflammation.
Introduction
Since the 1980s, the world’s population with obesity has doubled to 13%, affecting more than 600 million adults and 42 million children under age 5 years in 2014 according to the World Health Organization. In the USA, more than 33% of adults and about 17% of youth have obesity; the prevalence has been stable over the past few years (1). Obesity is associated with glucose intolerance and insulin resistance, dyslipidemia, increased inflammation, and oxidative stress. Obesity is also an important factor contributing to hypertension, cardiovascular disease, type 2 diabetes, stroke, and cancer; thus, obesity is an underlying cause of numerous potentially preventable deaths. Early prevention or treatment is needed to delay the development of these diseases; however, effective strategies for addressing childhood obesity have yet to be proven.
Recent results of long-term follow-up studies have suggested that increased body mass index accounts for a 20% increment in the incidence of renal disease (2). Severe obesity increases renal blood flow, glomerular filtration rate, and albumin extraction rate. Pathological changes of obesity-related glomerulopathy (ORG) include glomerular hypertrophy, mesangial matrix accumulation, and secondary focal segmental glomerulosclerosis both in humans and animals (3). The typical symptom of ORG is slowly progressive proteinuria, from microalbuminuria to proteinuria and then loss of renal function, leading to end-stage renal disease. The mechanisms of ORG are not fully understood.
The mitogen-activated protein kinases (MAPKs) are a family of serine/threonine kinases mainly containing extracellular signal-regulated protein kinases, c-Jun N-terminal kinases, and P38 MAPK. Previous studies have found that the activation of P38 MAPK can cause increased cellular hypertrophy, apoptosis, and proliferation, along with increasing inflammation in heart and liver (4–6). P38 MAPK also regulates mesangial cell proliferation and matrix accumulation in the context of nephropathy under diabetic or nondiabetic conditions (7–9). These findings indicate that P38 MAPK may play a key role in the development and progression of ORG. Inactivating the P38 MAPK inflammatory pathway may pose an effective approach to preventing the development of ORG among individuals with obesity.
Zinc (Zn) is a trace element that functions as a cofactor for catalytic enzymes essential to biological functions. Zn has been described in association with the structural and functional integrity of more than 2000 transcription factors. Zn supplementation affords benefits both in animal models and patients with obesity and/or diabetic conditions (10–12). We demonstrated that Zn protects against diabetes-induced pathogenic effects in the heart, liver, and kidney through increasing insulin sensitivity, and diminishing anti-oxidative stress (13–15). Additional studies about the effects of Zn on ORG are needed.
Zn deficiency is associated with diabetes, obesity, and aging (16–19). Zn deficiency also actives P38 MAPK-mediated inflammation (20). We hypothesized that Zn deficiency may accelerate the development and progression of ORG. We further hypothesized that Zn supplementation or adequate Zn intake may prevent or mitigate ORG by suppressing the P38 MAPK pathway. To test these hypotheses, we used high-fat diet (HFD)-induced obesity and ORG models with just-weaned young mice. To explore the role of Zn and P38 MAPK in the development or progression of ORG, we provided mice with normal diet (ND) and HFD containing three levels of Zn: deficient, adequate, and supplemented. We also implemented a treatment study arm with P38 MAPK inhibitor.
Methods
Animals
Four weeks (in the first study) and 28 weeks (in the second study) old C57BL/6J male mice were purchased from the Jackson Laboratory (Bar Harbor, Maine). Mice were kept in the University of Louisville Research Resources Center with 12 h light to dark cycles at 22°C and provided with free access to food and water. All experimental procedures for animal usage were in accordance with the NIH Guide of the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the University of Louisville.
Obesity and Zn treatment models
In the first study, mice were fed with HFD (60% kcal from fat) and age-matched control mice were fed with ND (10% kcal from fat). Diets contained varying levels of Zn: 10 mg (mildly deficient, labeled as L), 30 mg (adequate, labeled as N) and 90 mg (supplement, labeled as H) Zn per 4057 kcal (D14020202, D14020201, D140203 for ND and D14020205, D14020204, D140206 for HFD, Research Diet, New Brunswick, NJ). Both ND- and HFD-feeding mice were, respectively, divided into 3 Zn status groups randomly, forming 6 subgroups, that is, ND/N.Zn, ND/L.Zn, ND/H.Zn, HFD/N.Zn, HFD/L.Zn, and HFD/H.Zn (n = 10 in each subgroup). Half of each subgroup (n = 5) were fed for 3 months and sacrificed, labeled as 3 M. The rest of the mice (n = 5) continued to be fed the same food for an additional 3 months (labeled as 6 M) and sacrificed after anesthetized by avertin intraperitoneal injection (300 mg/kg bodyweight).
P38 inhibited models
To define the role of P38 MAPK in the development of ORG, mice were randomly divided into five groups (n = 5): one group was fed with ND/N.Zn; two groups were given HFD/N.Zn; and the rest were given HFD/L.Zn. Both HFD feeding groups were further randomly divided into those with or without P38 MAPK inhibitor SB203580 (No. 1076, Selleck Chemicals, Houston, TX), labeled as HFD/N.Zn, HFD/N.Zn/SB, HFD/L.Zn, and HFD/L.Zn/SB, respectively. SB203580 was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO) and diluted in phosphate-buffered saline (Corning Cellgro, Manassas, VA) as working solution. Mice received SB203580 intraperitoneal injection at 1 mg/kg body weight or the same volume of vehicle every other day for 3 months, respectively. After 3 months of treatment, all mice were sacrificed.
Tissue Zn concentration assay
Each liver sample (100 mg wet-weight) was digested with 0.5 ml concentrated nitric acid overnight. After digestion, 1.5 ml deionized water (18.2 Ω) was added to each sample and the sample was placed into boiling water for 1 h. All digested samples were then filtered using a PTFE 0.2 μm filter. Liver Zn concentration was measured with Atomic Absorption Spectroscopy (AAS, an iCE-3000 AAS instrument from Thermo Scientific, Waltham, MA). Zn concentration was calculated based on a standard curve and shown as μg/mg wet tissue.
Renal function measurement
Mice spot urine samples were collected to measure and calculate the urinary albumin-to-creatinine ratio (UACR) as described before (21). Urinary albumin (E90–134, Bethyl Laboratories, Montgomery, TX) and creatinine (DICT-500, Bioassay Systems, Hayward, CA) assay kits were performed according to the manufacturers’ instructions. All tests were repeated three times.
Histology, immunohistochemistry, and immunofluorescence staining
Kidney cryo-sections were used for Oil-Red-O staining and paraffin sections for periodic acid-Schiff (PAS) staining, immunohistochemistry (IHC) staining, and immunofluorescence (IF) staining following published papers and as listed in Supporting Information (21,22).
RNA isolation and real-time polymerase chain reaction
Real-time polymerase chain reaction (RT-PCR) was performed following our published methods (21) and details were provided in Supporting Information.
Western blot assay
The Western blot was performed following our published articles (21) and details were presented in Supporting Information.
Cell cultures
HK-II cells were treated with palmitic acid (PA) to mimic the condition in vivo following our published work (15) with details provided in Supporting Information.
Statistical analysis
Data were presented as mean ± standard deviation (S.D.). Statistical significance was considered as P < 0.05. Details were described in Supporting Information following previously published work (21).
Results
Zn delayed obesity-induced renal hypertrophy and disorders
HFD feeding induced significant time-dependent increments in body weight, blood glucose, plasma triglyceride and insulin levels (not shown). Representing the tissue Zn level, hepatic Zn was significantly increased in ND/H.Zn and HFD/H.Zn-fed mice and slightly decreased in ND/L.Zn and HFD/L.Zn mice (Figure 1A).
Figure 1.
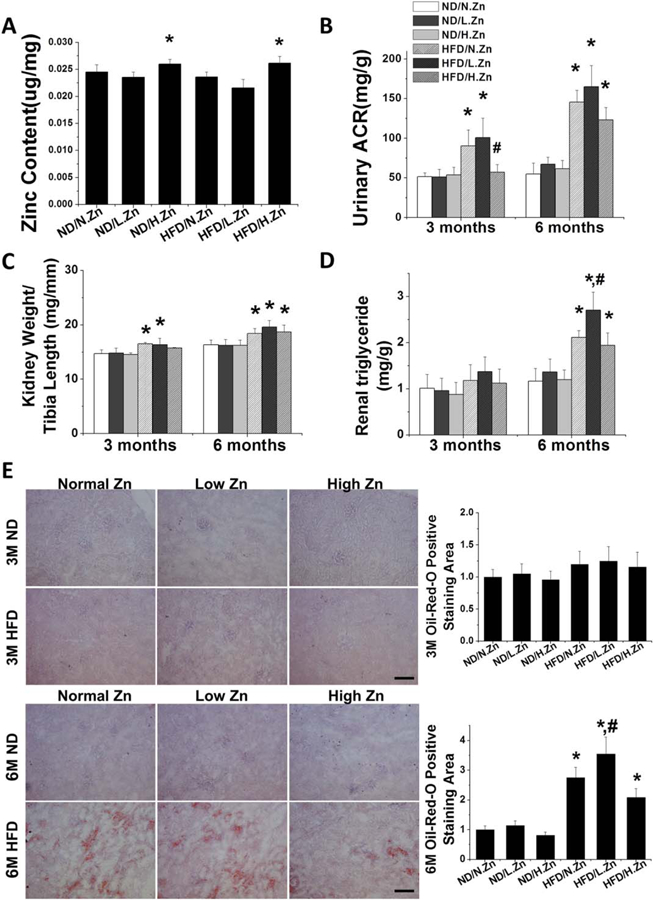
Zn delayed obesity-induced renal hypertrophy and dysfunction. Obesity was induced by feeding HFD with different concentrations of Zn (10/30/90 mg Zn per 4057 kcal) for 3 and 6 M. (A) Liver Zn concentration was measured by atomic absorption spectroscopy as a reference of animal tissue Zn level at 6 M. (B) Ratio of kidney weight to tibia length was measured and calculated, respectively. (C) Renal function was evaluated by calculating the UACR of spot urine samples at 3 and 6 M. (D) Kidney triglyceride concentration was assayed both in 3 and 6 M. (E) Kidney lipid infiltration and accumulation were examined by Oil-Red-O staining (200×, scale bar 200 μm) and semiquantitative analysis for lipid accumulation (red area) per image was counted in 10 vision fields across the kidney. Data are presented as the mean ± S.D. (n≥5). *, P < 0.05 vs. corresponding ND/N.Zn; #, P < 0.05 vs. corresponding HFD/N.Zn.
Based on UACR (Figure 1B), renal function was significantly decreased in HFD/N.Zn and HFD/L.Zn groups at both the 3 and 6 M time points Zn supplementation prevented HFD-induced renal disorders completely at the 3 M time point but only slightly at the 6 M time point (Figure 1B).
The ratio of kidney weight to tibia length was significantly increased in HFD/N.Zn and HFD/L.Zn groups at both the 3 and 6 M time points (Figure 1C). Zn supplementation prevented the rise of this ratio at 3 M, but not at 6 M.
Kidney triglyceride content (Figure 1D) and lipid accumulation, shown by Oil-Red-O staining (Figure 1E), was increased in all HFD groups at the 6 M time point Zn deficiency significantly worsened and Zn supplement slightly (no statistical difference) improved HFD-increased lipid effects. These results suggest that Zn deficiency accelerated obesity-induced renal lipid accumulation, hypertrophy, and disorders while Zn supplementation delayed the pathological progression without significantly influencing renal lipid metabolism.
Zn delayed obesity-induced glomerular area expansion, mesangial matrix accumulation, and tubular damage
The general morphologic changes of kidney were examined by PAS staining (Figure 2A) followed by semiquantitative analysis (Figure 2B). At the 3 M time point, the PAS positive staining areas were significantly increased in the HFD/N.Zn group and further increased in the HFD/L.Zn group. These effects could be almost completely prevented by Zn supplementation. At the 6 M time point, PAS materials were significantly increased in both HFD/N.Zn and HFD/H.Zn groups and further increased in the HFD/L.Zn group, indicating a time-dependent increment. The glomerular area in each group was not significantly different from each other at the 3 M time point. At 6 M, the glomerular area was increased similarly in HFD/N.Zn and HFD/H.Zn groups and further increased in HFD/L.Zn (Figure 2C). Tubular areas were examined (Figure 2D) by analysis of tubular cell vacuolization. Similar with glomeruli, HFD damaged renal tubules. Zn deficiency worsened and Zn supplementation delayed the pathology progression, respectively.
Figure 2.
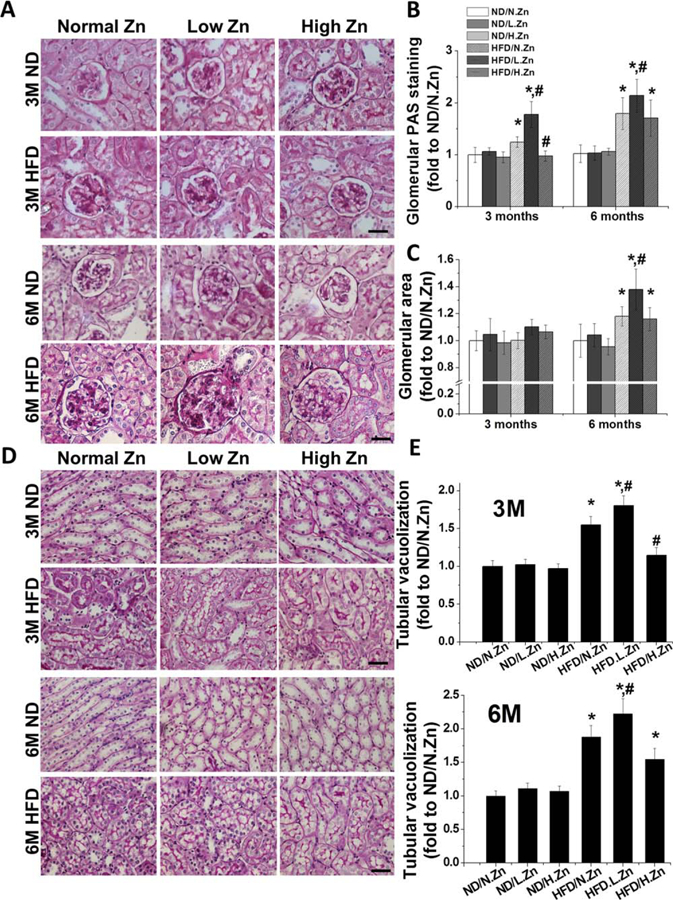
Zn delayed obesity-induced glomerular area expansion, matrix accumulation, and tubular damage. (A) Renal morphological change and mesangial matrix accumulation (pink) were examined by PAS staining in kidney section (400×, scale bar 100 μm). (B) Semiquantitative analysis for PAS positive staining area. (C) Glomerular area was evaluated by measuring the area of 10 vision fields of glomeruli. (D) Morphology changes in tubular area of PAS staining (400×, scale bar 100 μm). (E) Semiquantitative analysis of tubular cell vacuolization levels. Data are presented as the mean ± S.D. after normalizing to ND/N.Zn (n≥5). *, P < 0.05 vs. corresponding ND/N.Zn; #, P < 0.05 vs. corresponding HFD/N.Zn.
The results demonstrate that Zn deficiency contributed to kidney injury. Zn supplementation prevented HFD-induced glomerular and tubular damage at 3 M, but not at 6 M.
Zn delayed obesity-induced glomerular cell proliferation and kidney inflammatory cytokine accumulation
Glomerular cell proliferation was examined by IHC of PCNA staining (Figure 3A), followed by semiquantitative analysis of incidence of glomerular PCNA positive cells (Figure 3B and 3C). At the 3 M time point the PCNA positive staining cells were significantly increased in the HFD group and further increased in the HFD/L.Zn group. Similar to the PAS staining, these increments could be completely blocked by Zn supplementation. At the 6 M time point the number of PCNA positive cells was significantly increased in both HFD and HFD/H.Zn groups and further increased in the HFD/L.Zn group with expanded glomerular area.
Figure 3.
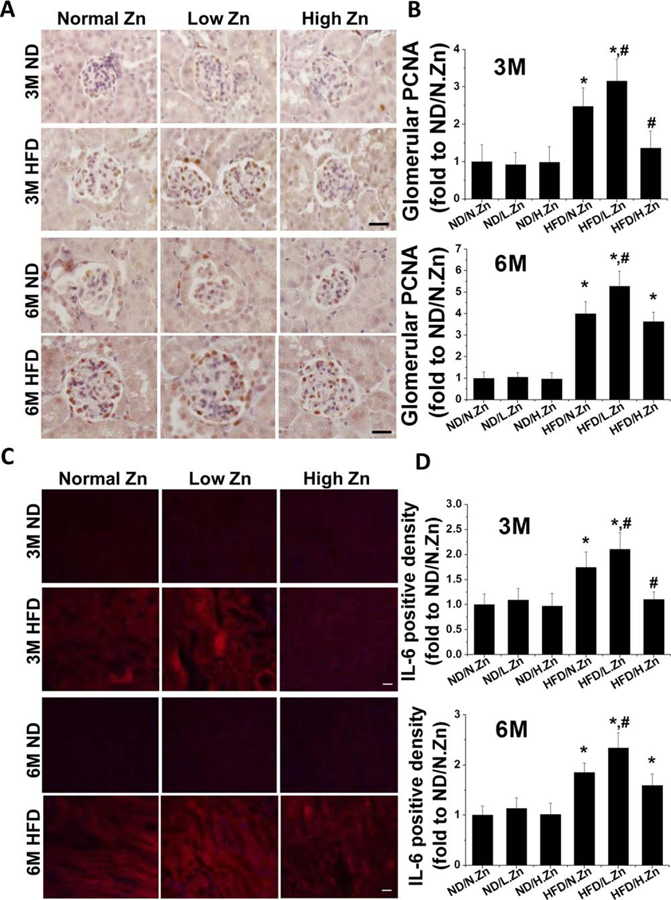
Zn delayed obesity-induced glomerular cell proliferation and tubular IL-6 cytokine accumulation. (A) Glomerular PCNA expression was examined by PCNA IHC staining (400×, scale bar 100 μm). (B) Semiquantitative analysis for the relative density of glomerular PCNA positive cells of 3 and 6 M. (C) IF of IL-6 in tubular area (200×, scale bar 100 μm). (D) This was followed by semiquantitative analysis of the positive IF staining of 3 and 6 M. Data are presented as the mean ± S.D. after normalizing to ND/N.Zn (n≥5). *, P < 0.05 vs. corresponding ND.N.Zn; #, P < 0.05 vs. corresponding HFD/N.Zn.
As no obviously positive fluorescent staining was seen in the glomerular area, IF staining for IL-6 in the tubular area and semiquantitative analysis of the positive staining area were examined. Similar to IHC staining of PCNA, the HFD group showed a time-dependent increment of IL-6 fluorescent positive area that worsened with Zn deficiency. HFD with Zn supplementation demonstrated protective function at 3 M but the protective effect did not persist until the 6 M time point.
The results indicated that Zn deficiency could contribute to glomerular cell proliferation and kidney inflammation. Zn supplementation prevented glomerular cell proliferation and inflammation at 3 M but not at 6 M.
Zn delayed kidney inflammation and activation of P38 MAPK induced by obesity
Expression of MCP-1, IL-1β, IL-6, and TNF-α at the mRNA level was evaluated by examining RT-PCR (Figure 3A–3D). All the inflammatory cytokines’ mRNA expression levels were progressively increased in the HFD group and exacerbated in the HFD/L.Zn group. The increased mRNA levels could be prevented at 3 M and alleviated at 6 M by Zn supplementation in the HFD/H-Zn group.
To further investigate renal inflammation induced by HFD, renal TNF-α protein levels were examined by Western blot (Figure 3E). Obesity significantly increased the protein expressions of TNF-α at 3 M in both the HFD group and the HFD/L.Zn group; obesity did not increase TNF-α in the HFD/H.Zn group at 3 M. At the 6 M time point, the TNF-α protein level was increased similarly in HFD and HFD/H.Zn groups but further increased in the HFD/L.Zn group, which indicated that Zn supplementation had waning anti-inflammation function.
The P38 MAPK pathway is crucial for inflammatory cytokine production and has been identified as having a pathogenic role in the progression of cell proliferation under environmental stress (9,23). Given increased renal inflammatory response and glomerular cell proliferation under HFD feeding, especially in the renal tubular area, we examined phosphorylated P38 (p-P38) MAPK levels using IHC and Western blot at 3 and 6 M time points (Figure 4F and 4G). The P38 MAPK phosphorylation levels increased progressively in the HFD group and this progression was more rapid in the HFD/L.Zn group. P38 MAPK phosphorylation was significantly delayed in the HFD/H.Zn group.
Figure 4.
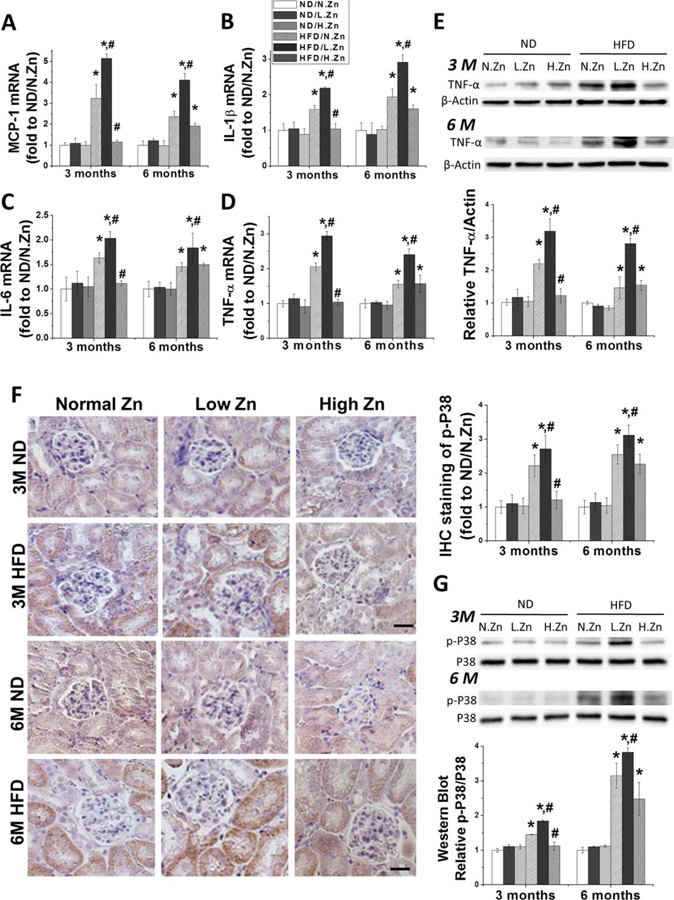
Zn inhibited the kidney inflammation response and the activation of P38 MAPK induced by obesity. (A–D) Renal mRNA level of MCP-1, IL-1β, IL-6, and TNF-α was detected by RT-PCR both in 3 and 6 M as the evaluation of renal inflammation response. (E) TNF-α protein expression level in kidney was examined by Western blot. (F) IHC staining of p-P38 MAPK of 3 and 6 M (400×, scale bar 100 μm). (G) Western blotting of p-P38 MAPK and total P38 MAPK after feeding the animals with different concentration of Zn. This was followed by the ratio of p-P38 to total P38 and IHC staining positive area of p-P38 to evaluate the activation of the P38 MAPK pathway. Data are presented as the mean ± S.D. after normalizing to ND/N.Zn (n≥5). *, P < 0.05 vs. corresponding ND/N.Zn; #, P < 0.05 vs. corresponding HFD/N.Zn.
The results indicated that Zn deficiency could exacerbate HFD-induced renal inflammation and the activation of P38 MAPK. Zn supplementation delayed renal inflammation from 3 to 6 M by affecting the P38 MAPK pathway.
Inhibition of P38 MAPK prevented renal disorders and hypertrophy induced by obesity with and without Zn deficiency
Data from the above studies revealed that Zn deficiency exacerbated and Zn supplementation ameliorated obesity-induced renal inflammation, hypertrophy, and disorders. These associations between Zn and renal damage were associated with activation of the P38 MAPK pathway at both 3 and 6 M time points. To investigate whether the mechanism for Zn-mediated renal protection from the obesity-induced renal pathological changes occurs through down-regulation of P38 MAPK, we performed a separate animal study using P38 MAPK specific inhibitor SB203580 to inhibit the P38 MAPK pathway.
UACR, as an index of renal dysfunction shown in Figure 1B, was increased and exacerbated among the HFD and HFD/L.Zn groups. Treatment with SB203580 completely prevented HFD-induced, and HFD/L.Zn-worsened, renal disorders (Figure 5A). The ratio of kidney weight to tibia length was significantly increased in HFD mice and further increased in HFD/L.Zn mice, and it was slightly prevented by SB203580 treatment (Figure 5B).
Figure 5.
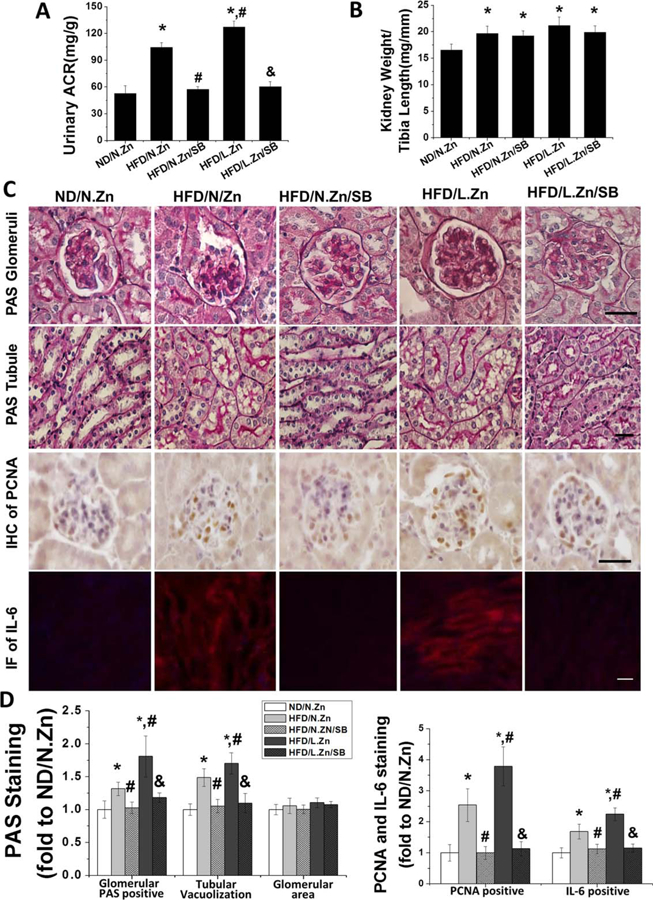
SB203580 prevented renal disorders and hypertrophy induced by obesity. After treatment with SB203580, mice kidney morphology changes induced by obesity were prevented. (A) Spot urine samples were collected to measure and calculate the UACR of SB203580 treated mice. (B) Ratio of kidney weight and tibia length was calculated. (C) PAS staining was performed to evaluate the general morphology changes of glomerular and tubular area. IHC staining of PCNA was assayed to detect the glomeruli cell proliferation. Tubular IF of IL-6 staining revealed the inflammation level of kidney. (D) Semiquantitative analysis of glomerular PAS positive staining area, tubular vacuolization levels, glomerular area, PCNA positive cells, and IF positive area (400×, scale bar 100 μm). Data are presented as the mean ± S.D. after normalizing to ND/N.Zn (n = 5). *, P < 0.05 vs. corresponding ND/N.Zn; #, P < 0.05 vs. corresponding HFD/N.Zn; &, P < 0.05 vs. corresponding HFD/L.Zn.
To further define kidney morphology changes, we conducted PAS staining, IHC staining for PCNA, and IF staining for IL-6 (Figure 5C), followed by semiquantitative analysis (Figure 5D). These assays revealed mesangial matrix accumulation, glomerular cell proliferation, tubular damage, and increased inflammation among HFD mice. These deleterious changes were further increased in the HFD/L.Zn group. Inhibition of P38 MAPK completely prevented these renal changes. These results suggest that P38 MAPK inhibition prevented HFD induced, and HFD/L.Zn worsened, renal disorders and hypertrophy.
SB203580 blocked the activation of the P38 MAPK pathway and prevented obesity-induced inflammation
To clarify whether SB203580 treatment could efficiently inhibit P38 MAPK activation, we conducted p-P38 MAPK IHC and Western blot assays of p-P38 and total P38 MAPK. These assays showed increased p-P38 MAPK level in the HFD group in the renal tubular area and even greater increased phosphorylation in the HFD/L.Zn group. The increased phosphorylation was almost completely abolished by SB203580 (Figure 6A), suggesting the efficient inhibition of P38 activation by SB203580.
Figure 6.
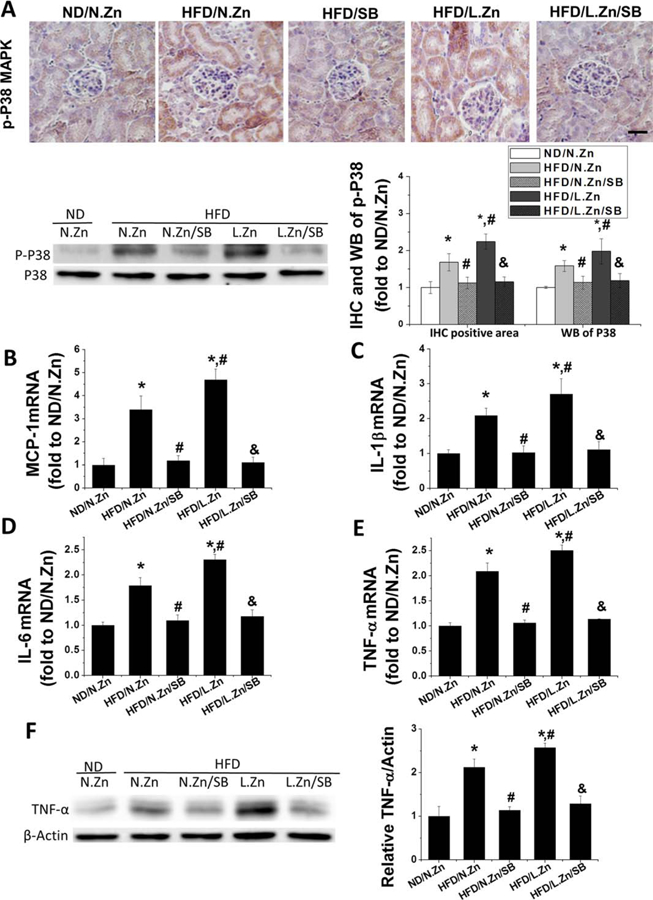
SB203580 blocked the activation of the P38 MAPK pathway and prevented obesity-induced inflammation. (A) The location of p-P38 MAPK was detected by IHC staining (400×, scale bar 100 μm), and its activation level of P38 MAPK was evaluated by performing Western blot. This was followed by the semiquantitative analysis of IHC positive area and the ratio of p-P38 to total P38 MAPK. (B–E): RT-PCR was used to detect the renal mRNA expression level of MCP-1, IL-1β, IL-6, and TNF-α as the evaluation of kidney inflammation. (F) Protein level of TNF-α was detected by Western blot. Data are presented as the mean ± S.D. after normalizing to ND/N.Zn (n = 5). *, P < 0.05 vs. corresponding ND/N.Zn; #, P < 0.05 vs. corresponding HFD/N.Zn; &, P < 0.05 vs. corresponding HFD/L.Zn.
RT-PCR analysis of renal mRNA expression of MCP-1, IL-1β, IL-6, and TNF-α showed increased levels of mRNA expression in the HDF group, with highest levels in the HFD/L.Zn group. Increased levels of mRNA expression were almost completely abolished by inhibition of P38 MAPK (Figure 6B–6E). The protein level of TNF-α was consistent with its mRNA profiles: significant increase in the HFD group and further increased in the HFD/L.Zn group, which were completely prevented by inhibition of P38 MAPK (Figure 6F).
Zn and SB203580 blocked the P38 MAPK activation and inflammation in renal tubular epithelial cells exposed to PA in vitro
As we observed the increased p-P38 MAPK and inflammation mainly located in the renal tubular area, HK-II cells were treated with PA to define whether FA as one of HFD key components could activate P38 MAPK and Zn or SB203580 treatment could inhibit P38 MAPK in renal intrinsic cells. A time-dependent increase of both p-P38 MAPK and TNF-α levels were observed with values peaking at 24 h (Figure 7A). Then we used HK-II cells treated with PA for 24 h, adding TPEN (to chelate free Zn), ZnCl2 or SB203580 respectively to mimic the in vivo Zn deficiency, Zn supplement and P38 MAPK inhibition conditions. The expression of p-P38 MAPK and TNF-α was increased in the PA treating group and further increased in the PA/TPEN group. The PA-increased p-P38 MAPK and TNF-α were almost completely prevented by Zn or SB203580 treatment (Figure 7B), suggesting that Zn and SB203580 could inhibit the activation of P38 MAPK and its downstream inflammatory cytokines in renal tubular cells.
Figure 7.
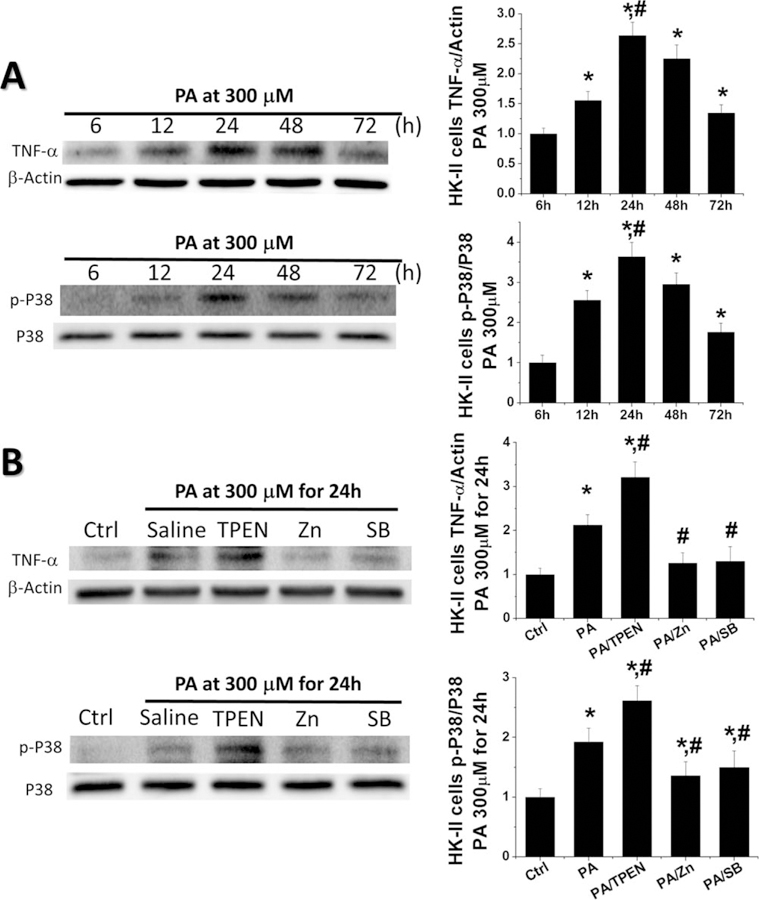
Zn and SB203580 blocked the activation of P38 MAPK and prevented PA-induced inflammation in renal tubular epithelial cells. To mimic the condition in vivo, HK-II cells were treated with PA alone for the indicated times or by adding TPEN, Zn, or SB203580 in some cultures, respectively. (A) Western blot was performed to detect the levels of TNF-α and the activation level of P38 MAPK at 6, 12, 24, 48, and 72 h. (B) Western blot was assayed to detect the levels of TNF-α and the activation level of P38 MAPK in PA treating cultures adding TPEN, Zn, or SB203580 for 24 h. Data are presented as the mean ± S.D. after normalizing to Ctrl (n = 5). *, P < 0.05 vs. corresponding Ctrl; #, P < 0.05 vs. corresponding PA.
Discussion
We provide the first evidence to show the preventive effect of Zn on ORG. Phosphorylation of P38 MAPK, renal inflammation, and kidney damage were all significantly increased in HFD mice. Zn deficiency exacerbated, and Zn supplementation significantly attenuated, P38 MAPK activation, inflammatory responses, and subsequent renal disorders. SB203580 blocked the P38 MAPK pathway and eliminated induced renal inflammation and damage in both HFD mice with or without Zn deficiency. These results suggest the importance of Zn in the prevention of HFD-induced P38 MAPK-mediated renal inflammation, and subsequent renal hypertrophy and disorders, as illustrated in Figure 8.
Figure 8.
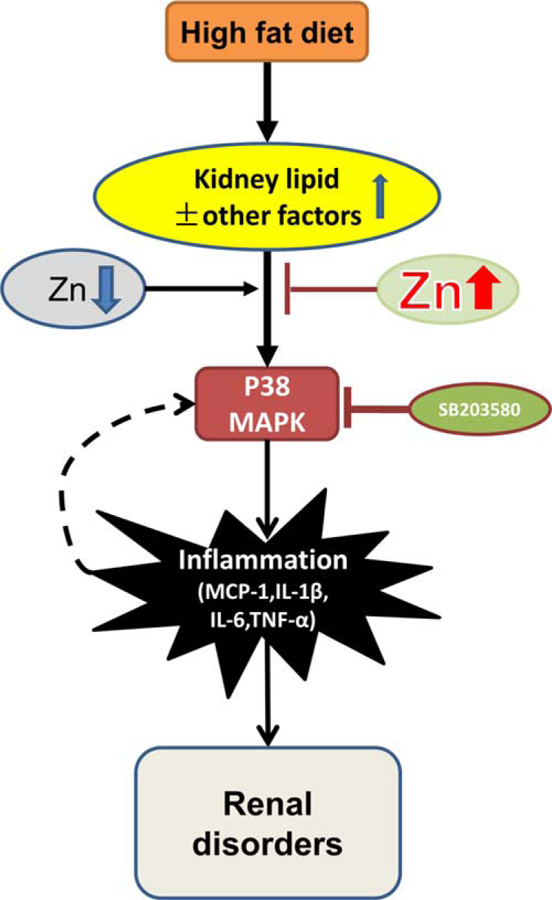
Schematic illustration for the effects of Zn on obesity-related nephropathy. Obesity induced lipid infiltration and accumulation in kidney, causing the activation of P38 MAPK, which led to renal cell proliferation, glomeruli hypertrophy, tubule damage, kidney remodeling, and renal disorder through inflammation. In the early stage of obesity-induced nephropathy, Zn could attenuate obesity-induced renal inflammation and disorders. However, with the pathological progression and continuous stimulation by inflammation induced by P38 MAPK, a feedback pathway of P38 MAPK-inflammation was formed, and glomeruli hypertrophy was detected. Zn lost its protective function at this stage. SB203580 could down-regulate the P38 MAPK pathway, leading to attenuated obesity- and Zn deficiency-induced inflammation, renal cell proliferation, and renal disorders. It indicated that Zn mediated renal protection from obesity was via down-regulating the P38 MAPK pathway in the early stage of obesity-induced nephropathy.
Although usually accompanied with diabetes and hypertension, obesity itself can induce chronic renal inflammation that initiates renal damage in both adults and children, reflecting in clinical symptoms as progressive proteinuria and loss of renal function (24). Limited clinic case reports showed that proteinuria was usually first detected and common in child ORG patients with or without hematuria, hyperuricemia, hypertension, and dyslipidemia. Renal biopsies of ORG patients have demonstrated glomeruli hypertrophy, proliferation of mesangial cells, and mesangial matrix expansion with or without segmental sclerosis (25,26); these histologic changes are similar with what we observed in the present study. Meta-analysis has shown that obesity could be associated with the development of renal dysfunction in childhood (27). Similar to adults, young people with obesity may be at high risk for renal dysfunction, although very few studies have been published.
ORG is associated with up-regulation of inflammatory mediators (28). Obesity is a chronic low-grade inflammatory condition, which eventually can lead to the development of metabolic disorders and renal disease (29,30). Although the adipose tissue is considered as a rich source of inflammatory cytokines (31), the renal intrinsic cell could develop inflammation (32), which were similar to what we observed in the study in vitro. Adipose mass increasing and lipid accumulation mediate the up-regulation of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β, and MCP-1) and cause kidney hypertrophy and dysfunction (33,34). Increasing numbers of inflammatory pathways have been described, in which the P38 MAPK pathway was found to have cross talk TNF-α pathways. P38 MAPK can be activated under environmental stresses and contributes to the production of inflammatory cytokines like TNF-α. Increased inflammatory cytokines reciprocally activate P38 MAPK pathways (35,36). Various inhibitors of P38 MAPK were found to have the anti-inflammation effects (5,37). We further confirmed these associations in this study, showing that P38 MAPK plays a major role in the development of ORG.
Zn deficiency commonly exists in diabetes and obesity patients (16,38) and Zn supplementation has beneficial effects on glucose and lipid control (12). In this study, HFD/L.Zn groups showed a progressive worsening of renal function along with increasing levels of inflammation and kidney damage. The effect of Zn supplementation on study mice kidney tissue differed by time. At 3 M, Zn supplementation showed significant anti-inflammatory protective function; however, there was no difference between HFD/H.Zn and HFD/N.Zn groups at 6 M. As we mentioned, Zn supplementation may have certain roles in the positive feedback crosstalk of P38 MAPK. Therefore, during the long period of exposure to HFD, several other undiscovered pathways may also involve in the development of ORG. How Zn affects these unknown pathways remains in need of further exploration.
Although supplementation of nutrients with Zn is very common and typically safe, Zn supplementation should be still considered in the context of its potential toxicity, particularly when supplementation occurs over longer durations. It has been reported that excessive Zn intake activated the renin-angiotensin system in rats, showing increased blood pressure and decreased renal blood flow and glomerular filtration rate when the rats were given Zn at doses of 50 mg/g (5-fold higher than our Zn supplementation) (39). In our study, the ND/H.Zn group displayed no significant renal dysfunction, indicating that our dose is not obviously toxic. However, under the HFD challenge, long-term Zn intake may cause the Zn to accumulate and exceed the safe threshold value at long-term (e.g., 6 M) time points. A limitation of the study is the extent to which inferences can be made on the animal model. Although the C57BL strain is sensitive to obesity, it is relatively resistant to renal injury. Studies have reported that C57BL mice develop mild kidney damage under HFD conditions (40), just like ours. As no animal models mimic disease in human populations perfectly, further studies about the HFD-induced ORG model are needed.
In conclusion, this is the first study to our knowledge that presents evidence regarding the relationships between HFD-induced renal disorders and long-term Zn treatment on young animal models. We confirm that Zn deficiency contributes to the pathological progression of kidney disorders induced by obesity and reveal the partially protective effect of long-term Zn supplementation on ORG. Study findings confirm the significant role of Zn in preventing obesity-related kidney disorders in mice models of child obesity. Further studies are required to identify the optimal Zn doses and timing of Zn supplementation. We interpret our research as revealing a strong potential for Zn supplements in the prevention and/or treatment of obesity-related kidney diseases.
Supplementary Material
Acknowledgments
The authors sincerely thank Dr. Shudong Wang and Dr. Lili Kong from the First Hospital of Jilin University and Dr. Jing Chen, Dr. Paul N. Epstein, and Dr. Madhavi Rane from University of Louisville for their help in feeding animals, technology supports, and providing the HK-II cell line.
Funding agencies: This research was supported in part by grants from the National Institutes of Health (1R01DK091338–01A1 and 3R01DK091338–02S1), the Natural Science Foundation of China (81170669, 81241023, and 81400725), and the National Key Technology R&D Program of China (2011BAI10B00).
Footnotes
Disclosure: The authors declared no conflict of interest.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Elia JA, Roshan B, Maski M, et al. Manifestation of renal disease in obesity: pathophysiology of obesity-related dysfunction of the kidney. Int J Nephrol Renovasc Dis 2009;2:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tefft KM, Shaw DH, Ihle SL, et al. Association between excess body weight and urine protein concentration in healthy dogs. Vet Clin Pathol 2014;43:255–260. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Liu JZ, Hu JX, et al. ROS-activated p38 MAPK/ERK-Akt cascade plays a central role in palmitic acid-stimulated hepatocyte proliferation. Free Radic Biol Med 2011;51:539–551. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Georgakopoulos D, Lu G, et al. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation 2005;111:2494–2502. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Huang S, Sah VP, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem 1998;273:2161–2168. [DOI] [PubMed] [Google Scholar]
- 7.Yan Z, Ni Y, Wang P, et al. Peroxisome proliferator-activated receptor delta protects against obesity-related glomerulopathy through the P38 MAPK pathway. Obesity (Silver Spring) 2013;21:538–545. [DOI] [PubMed] [Google Scholar]
- 8.Lee SJ, Kang JG, Ryu OH, et al. Effects of alpha-lipoic acid on transforming growth factor beta1-p38 mitogen-activated protein kinase-fibronectin pathway in diabetic nephropathy. Metabolism 2009;58:616–623. [DOI] [PubMed] [Google Scholar]
- 9.Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov 2003;2:717–726. [DOI] [PubMed] [Google Scholar]
- 10.Shidfar F, Aghasi M, Vafa M, et al. Effects of combination of zinc and vitamin A supplementation on serum fasting blood sugar, insulin, apoprotein B and apoprotein A-I in patients with type I diabetes. Int J Food Sci Nutr 2010;61:182–191. [DOI] [PubMed] [Google Scholar]
- 11.Afkhami-Ardekani M, Karimi M, Mohammadi SM, et al. Effect of zinc sulfate supplementation on lipid and glucose in type 2 diabetic patients. Pak J Nutr 2008; 7:550–553. [Google Scholar]
- 12.Jayawardena R, Ranasinghe P, Galappatthy P, et al. Effects of zinc supplementation on diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr 2012;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cong W, Zhao T, Zhu Z, et al. Metallothionein prevents cardiac pathological changes in diabetes by modulating nitration and inactivation of cardiac ATP synthase. J Nutr Biochem 2014;25:463–474. [DOI] [PubMed] [Google Scholar]
- 14.Liang T, Zhang Q, Sun W, et al. Zinc treatment prevents type 1 diabetes-induced hepatic oxidative damage, endoplasmic reticulum stress, and cell death, and even prevents possible steatohepatitis in the OVE26 mouse model: Important role of metallothionein. Toxicol Lett 2015;233:114–124. [DOI] [PubMed] [Google Scholar]
- 15.Li B, Cui W, Tan Y, et al. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J Cell Mol Med 2014;18:895–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marreiro DN, Fisberg M, Cozzolino SM. Zinc nutritional status and its relationships with hyperinsulinemia in obese children and adolescents. Biol Trace Elem Res 2004;100:137–149. [DOI] [PubMed] [Google Scholar]
- 17.Tungtrongchitr R, Pongpaew P, Phonrat B, et al. Serum copper, zinc, ceruloplasmin and superoxide dismutase in Thai overweight and obese. J Med Assoc Thai 2003; 86:543–551. [PubMed] [Google Scholar]
- 18.Isbir T, Tamer L, Taylor A, et al. Zinc, copper and magnesium status in insulin-dependent diabetes. Diabetes Res 1994;26:41–45. [PubMed] [Google Scholar]
- 19.Herbein G, Varin A, Fulop T. NF-kappaB, AP-1, Zinc-deficiency and aging. Biogerontology 2006;7:409–419. [DOI] [PubMed] [Google Scholar]
- 20.Zago MP, Mackenzie GG, Adamo AM, et al. Differential modulation of MAP kinases by zinc deficiency in IMR-32 cells: role of H(2)O(2). Antioxid Redox Signal 2005;7:1773–1782. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Wang Y, Luo M, et al. Novel curcumin analog C66 prevents diabetic nephropathy via JNK pathway with the involvement of p300/CBP-mediated histone acetylation. Biochim Biophys Acta 2015;1852:34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Wang S, Zhou S, et al. Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J Mol Cell Cardiol 2014;77:42–52. [DOI] [PubMed] [Google Scholar]
- 23.Lim AK, Nikolic-Paterson DJ, Ma FY, et al. Role of MKK3-p38 MAPK signalling in the development of type 2 diabetes and renal injury in obese db/db mice. Diabetologia 2009;52:347–358. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava T Nondiabetic consequences of obesity on kidney. Pediatr Nephrol 2006;21:463–470. [DOI] [PubMed] [Google Scholar]
- 25.Kawasaki Y, Isome M, Ono A, et al. Two children with obesity-related glomerulopathy identified in a school urinary screening program. Pediatr Int 2014; 56:115–118. [DOI] [PubMed] [Google Scholar]
- 26.Fowler SM, Kon V, Ma L, et al. Obesity-related focal and segmental glomerulosclerosis: normalization of proteinuria in an adolescent after bariatric surgery. Pediatr Nephrol 2009;24:851–855. [DOI] [PubMed] [Google Scholar]
- 27.Kaneko K, Kimata T, Tsuji S, et al. Impact of obesity on childhood kidney. Pediatr Rep 2011;3:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu ZG, Lanting L, Vaziri ND, et al. Upregulation of angiotensin II type 1 receptor, inflammatory mediators, and enzymes of arachidonate metabolism in obese Zucker rat kidney: reversal by angiotensin II type 1 receptor blockade. Circulation 2005; 111:1962–1969. [DOI] [PubMed] [Google Scholar]
- 29.Ouchi N, Parker JL, Lugus JJ, et al. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 2011;11:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egger G, Dixon J. Non-nutrient causes of low-grade, systemic inflammation: support for a ‘canary in the mineshaft’ view of obesity in chronic disease. Obes Rev 2011;12:339–345. [DOI] [PubMed] [Google Scholar]
- 31.El-Atat FA, Stas SN, McFarlane SI, et al. The relationship between hyperinsulinemia, hypertension and progressive renal disease. J Am Soc Nephrol 2004;15:2816–2827. [DOI] [PubMed] [Google Scholar]
- 32.Meng Q, Liu H, Wang J. Protective effects of polydatin on HK-2 cells against oxygen-glucose deprivation/re-oxygenation-induced injury by regulating Sonic hedgehog through PI3K/Akt signaling pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2015;31:1452–1457. [PubMed] [Google Scholar]
- 33.Decleves AE, Mathew AV, Cunard R, et al. AMPK mediates the initiation of kidney disease induced by a high-fat diet. J Am Soc Nephrol 2011;22:1846–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh SS, Gehr TW, Ghosh S, et al. PPARgamma ligand attenuates PDGF-induced mesangial cell proliferation: role of MAP kinase. Kidney Int 2003;64: 52–62. [DOI] [PubMed] [Google Scholar]
- 35.Palomer X, Alvarez-Guardia D, Rodriguez-Calvo R, et al. TNF-alpha reduces PGC-1alpha expression through NF-kappaB and p38 MAPK leading to increased glucose oxidation in a human cardiac cell model. Cardiovasc Res 2009;81:703–712. [DOI] [PubMed] [Google Scholar]
- 36.Matoba K, Kawanami D, Tsukamoto M, et al. Rho-kinase regulation of TNF-alpha-induced nuclear translocation of NF-kappaB RelA/p65 and M-CSF expression via p38 MAPK in mesangial cells. Am J Physiol Renal Physiol 2014;307:F571–F580. [DOI] [PubMed] [Google Scholar]
- 37.Adams JL, Badger AM, Kumar S, et al. p38 MAP kinase: molecular target for the inhibition of pro-inflammatory cytokines. Prog Med Chem 2001;38:1–60. [DOI] [PubMed] [Google Scholar]
- 38.Pidduck HG, Wren PJ, Evans DA. Hyperzincuria of diabetes mellitus and possible genetical implications of this observation. Diabetes 1970;19:240–247. [DOI] [PubMed] [Google Scholar]
- 39.Kasai M, Miyazaki T, Takenaka T, et al. Excessive zinc intake increases systemic blood pressure and reduces renal blood flow via kidney angiotensin II in rats. Biol Trace Elem Res 2012;150:285–290. [DOI] [PubMed] [Google Scholar]
- 40.van der Heijden RA, Bijzet J, Meijers WC, et al. Obesity-induced chronic inflammation in high fat diet challenged C57BL/6J mice is associated with acceleration of age-dependent renal amyloidosis. Sci Rep 2015;5:16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


