Fig. 4. Most commonly used assays for indirect assessments of different “peroxidation/peroxidase activities associated with the execution of ferroptosis. Note that only Liperfluo assay detects hydroperoxyl-lipids.
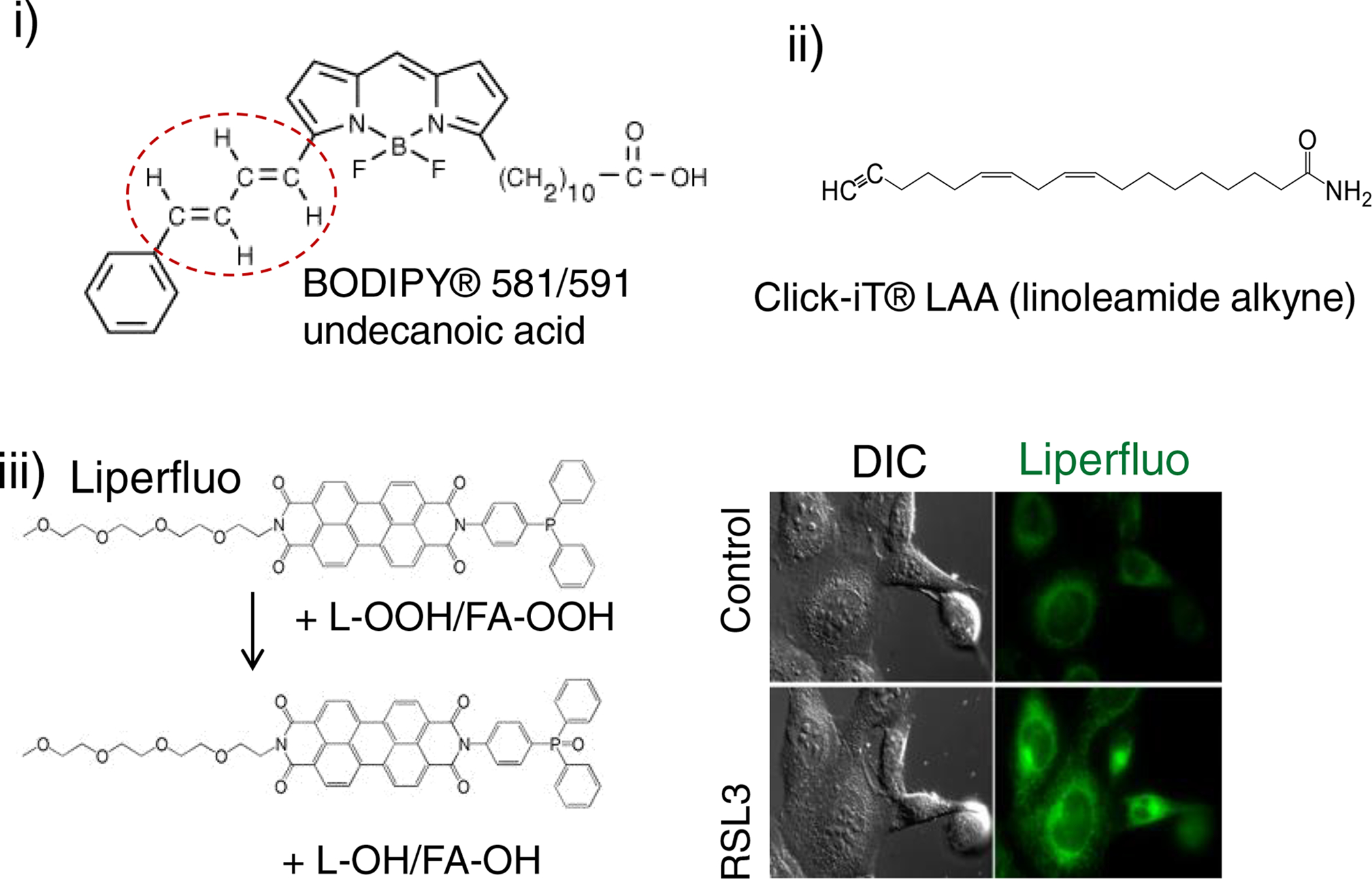
i) BODIPY® 581/591 detects “peroxidase activity” resulting in changed fluorescence characteristics of the non-lipidic BODIPY chromophore. Oxidation of butadiene portion (circled in red) of the BODIPY® 581/591 results in a shift of fluorescence emission peak from 590 nm to 510 nm.
ii) linoleate-based Click-iT® LAA (linoleamide alkyne) assay. This assay is designed to detect lipid-peroxidation derived modification of protein in fixed cells. Incorporated into cellular membranes Click-iT® LAA can undergo lipid peroxidation resulting in production of 9- and 13-hydroperoxy linoleic acid that further decomposes to unsaturated aldehydes which can modify proteins. These modified proteins are detected by Click-iT® chemistry.
iii) Liperfluo fluorogenic assay of hydroperoxy-containing groups. Liperfluo, a perylene derivative containing oligooxyethylene, detects L-OOH. LiperFluo can react with L-OOH and its fluorescence reliably reports intracellular sites of L-OOH accumulation by a fluorescence microscopy. Both free PUFA-OOH and PUFA-OOH esterified into phospholipids display high reactivity toward LiperFluo. The results in the robust fluorescence response of oxidatively modified LiperFluo in cells exposed to pro-ferroptotic stimuli) are shown on the right panel. Chemically, Liperfluo reduces L-OOH to the respective alcohols similarly to the reaction catalyzed by GPX4.
