Abstract
Recent findings support positive effects of acute aerobic exercise on worry, state anxiety, and feelings of energy and fatigue among young adult women with subclinical, or analogue, generalized anxiety disorder (GAD). However, exercise effects among young adult men with analogue GAD are unstudied.
Purpose:
This study replicated initial findings of positive effects of acute vigorous-intensity aerobic exercise on worry, state anxiety, and feelings of energy and fatigue among young adult women with analogue GAD, examined responses among young adult men with analogue GAD, and explored sex-related differences and moderation by physical activity level, trait anxiety, depression, and poor sleep status.
Methods:
Thirty-five young adults (21.4 ± 2.3 yr; 19 males; 16 females) with Penn State Worry Questionnaire scores ≥45 (60 ± 8) completed two counterbalanced 30-min conditions: treadmill running at ~71.2% ± 0.04% HR reserve and seated quiet rest. Outcomes included worry, worry engagement, absence of worry, state anxiety, and feelings of energy and fatigue.
Results:
No outcome or moderator differed at baseline between sexes. Exercise significantly improved state anxiety (P < 0.04; d = 0.27) and feelings of energy (P < 0.001; d = 1.09). Small nonsignificant improvements were found for worry (d = 0.22), worry engagement (d = 0.18), and feelings of fatigue (d = 0.21). The magnitude of improvements in worry, worry engagement, absence of worry, and feelings of energy were stronger among females. Significant large, potentially clinically meaningful increases in feelings of energy were found among women (d = 1.35) and men (d = 0.92). A nonsignificant, but potentially clinically meaningful, moderate reduction in worry (d = 0.53) was found among women. High-trait anxiety and poor sleep quality were supported as moderators.
Conclusions:
Findings replicated positive effects of acute aerobic exercise among young adult women with analogue GAD, and extended to support for positive effects among young adult men with analogue GAD
Keywords: ACUTE EXERCISE, WORRY, ANXIETY, FATIGUE, ENERGY, GENERALIZED ANXIETY DISORDER
Previous evidence supported positive effects of both aerobic and resistance exercise training among young adult women with generalized anxiety disorder (GAD), including improved clinical severity (1), sleep quality and quantity (2), quality of life (3), and associated symptoms (e.g., worry, anxiety, and feelings of energy and fatigue) (4). More recent evidence highlighted the benefits of a single bout of aerobic exercise among a small sample of young women with subclinical, or analogue, GAD (i.e., elevated worry, the hallmark of GAD) (5). Compared with a 30-min bout of seated rest, a 30-min bout of vigorous-intensity treadmill running completed at ~73% HR reserve significantly improved worry engagement, state anxiety, and feelings of energy and fatigue (5). A quarter standard deviation improvement for worry, approximating a 3.5% change, and moderate-to-large improvements in state anxiety and feelings of energy and fatigue were found (5).
People who report elevated symptom scores are more likely to develop clinically significant psychopathologies (6). It is plausible that exercise-induced attenuation of elevated symptoms may reduce the likelihood of symptom persistence and exacerbation. Thus, investigating exercise effects on the symptom profile of GAD and identifying traits that moderate these effects among individuals with analogue GAD has the potential to inform precision medicine prevention strategies. To the authors’ knowledge, the initial findings of positive effects of acute exercise among females with analogue GAD has neither been replicated, nor extended to males.
Lifetime prevalence of GAD is nearly twice as high among women (7), who also have a higher likelihood of reporting symptoms that have been improved by exercise (i.e., fatigue, irritability, muscle tension, and somatic symptoms) (4,8). Therefore, it is perhaps not surprising that the effects of acute exercise on worry, state anxiety, and feelings of energy and fatigue among young adult men have not been investigated. However, GAD is also prevalent among young adult men, and evidence supports small-to-moderate positive effects of acute exercise on state anxiety and feelings of energy and fatigue among otherwise healthy young adult men (9).
Thus, this study tested the effects of acute aerobic exercise compared to quiet rest on worry, state anxiety, and feelings of energy and fatigue among young adult women and men with worry indicative of analogue GAD to: 1) replicate and extend initial positive findings among young adult women with analogue GAD to include young adult men with analogue GAD; 2) to explore potential sex-related differences; and, 3) to replicate preliminary findings that elevated trait anxiety, depression, and poor sleep status moderate responses to exercise or quiet rest conditions. The authors hypothesized that, compared to quiet rest, an acute bout of aerobic exercise would significantly improve worry, state anxiety, and feelings of energy and fatigue among young adult women and men with worry indicative of GAD.
METHODS
Design and Participants
The methods reported here are consistent with the authors’ previously reported acute aerobic exercise studies (5,9). The study protocol was approved by the University’s Research Ethics Board. Before participation, interested potential participants provided written informed consent and completed a medical history screening questionnaire that included the Physical Activity Readiness Questionnaire. Potential participants were screened based on the Penn State Worry Questionnaire (PSWQ) (10). Inclusion criteria were: (i) PSWQ score ≥45, which has demonstrated high sensitivity and specificity as a cut-score to identify individuals with elevated worry indicative of GAD (11), (ii) age 18–35 yr; (iii) no medical contraindication to safe participation in vigorous aerobic exercise, and (iv) no current pregnancy or lactation. Self-reported physical activity indicative of an active lifestyle was not an exclusion criterion. Of 64 individuals consecutively recruited from the University and surrounding communities using leaflets, recruitment emails to approved listservs, and word-of-mouth, 35 young adults (19 male; 16 female), age 21.4 ± 2.3 yr, met inclusion criteria. Based on power analysis performed with G*Power, a sample size of 35 would provide >80% statistical power to detect differences in worry assuming a two-tailed α = 0.05, a correlation between repeated measures of r = 0.8, and a small effect of exercise on worry (f = 0.13, d = 0.26) based on previous evidence (1,5,9). Participants were randomized (www.randomizer.org) by a researcher who did not perform assessments to complete two conditions in counterbalanced order at approximately the same time of day, with approximately 48 h between conditions: (i) 30 min of vigorous treadmill running, or (ii) 30 min seated quiet rest (5,9,12).
Baseline Measures
Before testing on day 1, each participant completed electronic versions (www.surveymonkey.com) of a Seven Day Physical Activity Recall (13), the trait subscale of the State-Trait Anxiety Inventory (STAI-Y2) (14), the Quick Inventory of Depressive Symptomatology (QIDS) (15), and the Pittsburgh Sleep Quality Index (PSQI) (16). Established cut-scores were used to classify high-trait anxious status (STAI-Y2 ≥ 50), depression status (QIDS ≥ 6 (15)) and poor sleep quality (PSQI > 5 (16)).
Outcomes
Using laboratory desktop PCs, immediately before and 10 min after exercise or quiet rest participants completed electronic assessments (www.surveymonkey.com) of worry, state anxiety, and the intensity of feelings of energy and fatigue. This process required approximately 10 to 15 min.
Worry.
Worry was assessed using the 16-item PSWQ (10). Item responses are summed after reverse scoring; total scores range from 16 to 80. Worry engagement (11 items worded in the direction of pathological worry) and absence of worry (5 items reverse-worded to combat acquiescence) subscales were also calculated (10,17); higher scores indicate worse symptom severity. The PSWQ demonstrated adequate internal consistency (α = 0.85), and correlations between repeated measures were r = 0.69 and r = 0.89 for exercise and quiet rest, respectively. Worry engagement demonstrated similar internal consistency (α = 0.86), and correlations between repeated measures were r = 0.86 and r = 0.67 for exercise and quiet rest, respectively. Internal consistency for absence of worry was poor (α = 0.57), and correlations between repeated measures were lower for both exercise (r = 0.64) and quiet rest (r = 0.60). Recent evidence supported that the PSWQ is sensitive to change in response to acute aerobic exercise (5,9).
State anxiety.
State anxiety was measured with the 20-item state subscale of the State-Trait Anxiety Inventory (STAI-Y1) (14). Item responses are summed after reverse scoring; total scores range from 20 to 80. The STAI-Y1 demonstrated adequate internal consistency (α = 0.94), and correlations between repeated measures were r = 0.80 and r = 0.92 for exercise and control, respectively.
Feelings of energy and fatigue.
Feelings of energy and fatigue were measured with the vigor and fatigue subscales of the Profile of Mood States—Brief Form. These subscales reliably measure the intensity of feelings of energy and fatigue (18,19). Participants were instructed to respond “based on how you feel RIGHT NOW.” Subscale scores range from 0 to 20. Internal consistency was adequate for fatigue (α = 0.84) and vigor (α = 0.88). For the fatigue subscale, correlations between repeated measures were r = 0.41 and r = 0.75 for exercise and control, respectively. For the vigor subscale, correlations between repeated measures were r = 0.50 and r = 0.81, respectively.
Conditions
Acute aerobic exercise.
Participants completed a vigorous (20) 30-min supervised bout of running on a Woodway Pro© treadmill at 65% to 85% of maximal HR reserve (%HRR). Intensity was estimated as a function of predicted maximal HR and an age-related norm resting HR of 73 bpm for females and 63 bpm for males. This method has demonstrated validity for estimating vigorous-intensity exercise dose in field-based exercise studies (21). Polar FT60© HR monitors were used to set the HR training zone and to continuously monitor HR and in-zone exercise minutes during exercise. Each participant performed a 5-min warm-up, continued running while investigators progressively increased the treadmill speed and/or grade to achieve 65% HRR, and then completed 30 min at a HR between 65% and 85% HRR. Most participants achieved 65% HRR within 5 min; therefore, warm-up periods ranged from 1 to 5 min. After completing 30 min at 65% to 85% HRR, each participant walked until sufficiently cooled down. Again, some participants cooled down more rapidly than others; therefore, the cooldown period ranged from 1 to 5 min. After completion of cooldown, each participant provided a session RPE for the full exercise bout using Borg 6 to 20 RPE scale (22). Standard instructions for RPE were provided to each individual. Muscle pain intensity was assessed with the 0 to 10 pain intensity scale developed and validated by Cook and colleagues (23). Given the potential mood effects of social interaction (24), extraneous conversation was minimized during the exercise session.
Control: Seated quiet rest.
Each participant completed 30 min of seated quiet rest in the same laboratory. Procedures were consistent with the exercise session except that each participant sat in an upright chair in a quiet area for 30 min. Participants were not allowed to read or listen to music given the potential for altered mood responses.
Analyses
Data analyses were performed using SPSS 22.0. Missing data for state anxiety (n = 5), worry (n = 6), feelings of fatigue (n = 4), and feelings of energy (n = 4) were imputed by multiple imputation: gender and time-variant responses for each variable were entered as predictors into separate multiple linear regression models and predicted values were retained. Independent samples t-tests examined baseline differences in outcomes based on gender and high-trait anxious, depression, and poor sleep status.
Two condition (exercise/quiet rest) × two time (pre/post) repeated measures ANOVA examined differences between exercise and quiet rest. Significant interactions were decomposed with simple effects analyses Bonferroni-corrected for multiple testing. Given increased calls to move beyond null-hypothesis significance testing in favor of effect sizes and confidence intervals (25), we also quantified and compared the magnitude of outcome change using Hedges’ d and associated 95% confidence intervals (95% CI) such that improved outcomes resulted in positive effect sizes (26). Effect sizes were judged as small (0.2), moderate (0.5), and large (0.8) based on Cohen’s thresholds.
Potential sex-related differences were examined using two group (male/female) × two condition (exercise/quiet rest) × two time (pre/post) repeated measures ANOVA. Hedges’ d (95% CI) quantified magnitude ofoutcome change for males and females.
Independent samples t-tests of pre–post exercise and pre–post quiet rest change scores analyzed within-condition differences in outcomes based on high-trait anxious status, depression status, and poor sleep status. Pearson’s correlation coefficients quantified associations between physical activity and outcome change scores for each condition.
RESULTS
Baseline Characteristics and Differences
Table 1 presents baseline participant characteristics.
TABLE 1.
Baseline participant characteristics.
| Male (n = 19) | Female (n = 16) | Total (N = 35) | |
|---|---|---|---|
| Age (yr) | 21.6 ± 1.2 | 21.2 ± 3.2 | 21.4 ± 2.3 |
| Weight (kg) | 78.7 ± 8.3 | 65.4 ± 9.0 | 72.8 ± 10.8 |
| BMI (kg·m−2) | 24.1 ± 1.8 | 23.1 ± 2.8 | 23.6 ± 2.3 |
| Overweight (%) | 5 (26.3%) | 3 (18.8%) | 8 (22.9%) |
| Physical Activity (kcal·wk−1) | 284.4 ± 45.4 | 293.3 ± 35.7 | 288.5 ± 40.9 |
| Moderate-to-vigorous physical activity (min·wk−1) | 811.6 ± 583.3 | 864.4 ± 463.4 | 835.7 ± 525.0 |
| Sleep (PSQI) | 5.5 ± 2.7 | 5.7 ± 2.9 | 5.6 ± 2.7 |
| Poor sleeper (PSQI > 5; %) | 6 (31.6%) | 9 (56.3%) | 15 (42.9%) |
| High-trait anxious (%) | 3 (15.8%) | 7 (43.8%) | 10 (28.6%) |
| Depressed (QIDS > 5; %) | 9 (47.4%) | 10 (62.5%) | 19 (54.3%) |
| None (%) | 10 (52.6%) | 6 (37.5%) | 16 (45.7%) |
| Mild depression (%) | 8 (42.1%) | 6 (37.5%) | 14 (40.0%) |
| Moderate depression (%) | 1 (5.3%) | 4 (25.0%) | 5 (14.3%) |
| Trait anxiety (STAI-Y2) | 41.9 ± 7.8 | 47.8 ± 10.8 | 44.6 ± 9.6 |
| State anxiety (STAI-Y1) | 35.6 ± 6.6 | 43.6 ± 15.3 | 39.3 ± 12.0 |
| Feelings of Energy (POMS-B) | 8.8 ± 3.6 | 6.6 ± 4.5 | 7.8 ± 4.1 |
| Feelings of Fatigue (POMS-B) | 3.5 ± 2.5 | 5.3 ± 3.3 | 4.3 ± 3.0 |
| Depression (QIDS) | 5.7 ± 3.4 | 7.4 ± 3.8 | 6.5 ± 3.7 |
| Worry (PSWQ) | 57.6 ± 8.0 | 62.6 ± 8.1 | 59.9 ± 8.3 |
| Worry Engagement (PSWQ) | 38.5 ± 7.3 | 41.9 ± 6.1 | 40.1 ± 6.9 |
| Absence of Worry (PSWQ) | 19.1 ± 2.3 | 20.7 ± 3.1 | 19.8 ± 2.8 |
BMI, body mass index; POMS-B: Profile of Mood States-Brief.
Sex-related differences.
No outcome or potential moderator significantly differed between sexes at baseline: worry (t33 = 1.80, P > 0.08), worry engagement (t33 = 1.46, P > 0.15), absence of worry (t27.3 = 1.67, P > 0.10), state anxiety (t19.6 = 1.94, P > 0.06), and feelings of energy (t33 = 1.86, P > 0.12) and fatigue (t33 = 1.80, P > 0.07), prior week physical activity (t33 = 0.63, P > 0.53), self-reported minutes of moderate-to-vigorous physical activity (t33 = 0.29, P > 0.77), STAI-Y2 (t26.81 = 1.81, P > 0.08), high-trait anxious status (X1 = 3.33, P > 0.06), PSQI total score (t32 = 0.20, P > 0.84), and poor sleep quality status (X1 = 1.80, P > 0.17).
Differences based on high-trait anxious, depression, and poor sleep status.
High-trait anxious participants reported significantly higher worry (t33 = −3.77, P < 0.001), worry engagement (t33 = −4.11, P < 0.001), state anxiety (t11.5 = −5.32, P < 0.001), and feelings of fatigue (t11.2 = −2.36, P < 0.03). Depressed participants reported significantly higher state anxiety (t33 = −3.22, P < 0.003) and feelings of fatigue (t27.97 = −3.00, P < 0.006). Poor sleepers reported significantly higher worry (t32 = −2.38, P < 0.03) and absence of worry (t32 = −3.27, P < 0.003).
Exercise Session Variables
Participants averaged 35.8 ± 2.4 min of exercise, 30.4 ± 0.4 min in-zone. Participants exercised at ~71.2% ± 0.04% HRR, approximating an average session HR of 160.8 ± 5.2 bpm, an average session RPE of 13 ± 2, and an average session pain rating of 3 ± 2. All participants completed >30 min at 65% to 85% HRR; there was little variability in in-zone minutes, speed, or gradient, and differences in total exercise time and exact %HRR at which participants exercised were not significantly associated with outcome responses (all P > 0.09).
Effects of Exercise Compared with Quiet Rest
Table 2 presents descriptives, standardized mean differences, paired t-tests, and Hedges’ d (95% CI). Statistically significant condition-time interactions were found for state anxiety (F1,34 = 4.64; P < 0.04; d = 0.27; 95% CI, −0.20 to 0.74) and feelings of energy (F1,34 = 42.30, P < 0.001; d = 1.09; 95% CI, 0.59–1.59). State anxiety was significantly reduced by exercise (mean difference, −3.92; P ≤ 0.001), and significantly lower for exercise compared with quiet rest postcondition (mean difference, −4.62; P ≤ 0.004). Feelings of energy were significantly increased by exercise (mean difference, 2.86; P < 0.001), significantly decreased by quiet rest (mean difference, −1.82; P < 0.001), and significantly greater for exercise compared with quiet rest postcondition (mean difference, 3.94; P < 0.001). Condition-time interactions were not significant for worry (F1,34 = 1.46; P > 0.23; d = 0.22; 95% CI, −0.25 to 0.69), absence of worry (F1,34 = 0.01; P > 0.91; d = 0.03; 95% CI, −0.44 to 0.50), worry engagement (F1,34 = 0.80, P > 0.37; d = 0.18; 95% CI, −0.29 to 0.65), or feelings of fatigue (F1,34 = 0.75; P > 0.39; d = 0.21; 95% CI, −0.26 to 0.68). Significant main effects for time were found for worry (F1,34 = 6.11, P < 0.02) and worry engagement (F1,34 = 4.15, P < 0.05). Worry (mean difference, −1.85; P < 0.02) and worry engagement (mean difference, −1.14; P < 0.05) were significantly lower postcondition.
TABLE 2.
Precondition and postcondition means (SD), standardized mean differences (d), within-condition change, and Hedges’ d (95% CI).
| Pre-EX | Post-EX | SMD (d) | t | P | Pre-CON | Post-CON | SMD (d) | t | P | Hedges’ d (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Worry (PSWQ) | 61.0 ± 8.1 | 58.2 ± 10.8 | 0.34 | 2.08 | <0.05 | 60.2 ± 8.7 | 59.3 ± 9.1 | 0.11 | 1.30 | >0.20 | 0.22 (−0.25 to 0.69) |
| Worry Engagement (PSWQ) | 40.6 ± 7.0 | 38.9 ± 9.0 | 0.26 | 2.26 | ≤0.03 | 40.0 ± 7.1 | 39.5 ± 7.7 | 0.07 | 0.48 | >0.63 | 0.18 (−0.29 to 0.65) |
| Absence of Worry (PSWQ) | 19.8 ± 2.6 | 19.4 ± 3.0 | 0.18 | 1.16 | >0.25 | 20.0 ± 3.0 | 19.6 ± 3.0 | 0.14 | 0.89 | >0.38 | 0.03 (−0.44 to 0.50) |
| State Anxiety (STAI-Y1) | 37.9 ± 10.3 | 34.0 ± 9.9 | 0.38 | 3.62 | ≤0.001 | 39.3 ± 13.4 | 38.6 ± 13.8 | 0.05 | 0.72 | >0.47 | 0.27 (−0.20 to 0.74) |
| Feelings of Energy (POMS-B) | 6.9 ± 4.0 | 9.8 ± 3.7 | 0.71 | 4.35 | ≤0.001 | 7.7 ± 4.5 | 5.8 ± 4.0 | −0.41 | 4.10 | <0.001 | 1.09 (0.59 to 1.59) |
| Feelings of Fatigue (POMS-B) | 4.9 ± 3.9 | 4.2 ± 4.1 | 0.20 | 1.04 | >0.30 | 5.1 ± 4.1 | 5.2 ± 4.3 | −0.02 | −0.20 | >0.84 | 0.21 (−0.26 to 0.68) |
P values in bold emphasis are statistically significant at P < 0.05 based on paired t-tests.
Sex-related Differences
Table 3 presents descriptives and standardized mean differences across gender and condition. Sex-condition-time interactions were not significant for any outcome (all P > 0.11). As shown in Figure 1, compared with quiet rest, exercise resulted in a significant large increase in feelings of energy, nonsignificant moderate improvements in worry and absence of worry, and nonsignificant small reductions in worry engagement and state anxiety among women. Among men, compared to quiet rest, exercise resulted in significant large increases in feelings of energy and small-to-moderate reductions in feelings of fatigue, and nonsignificant small-to-moderate reductions in state anxiety. Effect sizes were larger among women than men for worry, worry engagement, absence of worry, and feelings of energy.
TABLE 3.
Precondition and postcondition means (SD), standardized mean differences (d), and Hedges’ d effect sizes (95% CI) for male and female participants.
| Male (n = 19) |
Female (n = 16) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-EX | Post-EX | SMD (d) | Pre-CON | Post-CON | SMD (d) | Hedges’ d (95% CI) | Pre-EX | Post-EX | SMD (d) | Pre-CON | Post-CON | SMD (d) | Hedges’ d (95% CI) | |
| Worry (PSWQ) | 57.7 ± 7.1 | 57.2 ± 10.0 | 0.07 | 58.0 ± 8.7 | 57.3 ± 9.6 | 0.08 | −0.02 (−0.66 to 0.61) | 64.9 ± 7.7 | 59.4 ± 11.9 | 0.71 | 62.9 ± 8.1 | 61.7 ± 8.1 | 0.15 | 0.53 (−0.17 to 1.24) |
| Worry Engagement (PSWQ) | 39.0 ± 6.7 | 38.1 ± 8.6 | 0.13 | 38.1 ± 7.3 | 37.9 ± 8.4 | 0.03 | 0.10 (−0.54 to 0.73) | 42.6 ± 7.0 | 39.8 ± 9.6 | 0.40 | 42.2 ± 6.2 | 41.3 ± 6.5 | 0.15 | 0.28 (−0.41 to 0.98) |
| Absence of Worry (PSWQ) | 18.7 ± 1.6 | 19.1 ± 2.3 | −0.25 | 19.4 ± 2.6 | 18.9 ± 2.6 | 0.19 | −0.41 (−1.05 to 0.23) | 21.2 ± 2.9 | 19.7 ± 3.8 | 0.52 | 20.7 ± 3.2 | 20.4 ± 3.4 | 0.09 | 0.38 (−0.32 to 1.08) |
| State Anxiety (STAI-Y1) | 34.6 ± 6.2 | 31.2 ± 5.1 | 0.55 | 35.5 ± 8.9 | 35.0 ± 9.7 | 0.06 | 0.37 (−0.27 to 1.01) | 41.9 ± 12.7 | 37.3 ± 13.0 | 0.36 | 43.8 ± 16.6 | 42.9 ± 16.7 | 0.05 | 0.24 (−0.45 to 0.94) |
| Feelings of Energy (POMS-B) | 8.4 ± 3.8 | 10.7 ± 3.1 | 0.61 | 8.3 ± 4.3 | 6.8 ± 4.1 | −0.35 | 0.92 (0.25 to 1.59)* | 5.1 ± 3.6 | 8.7 ± 4.2 | 1.00 | 6.9 ± 4.7 | 4.7 ± 3.6 | −0.47 | 1.35 (0.58 to 2.12)* |
| Feelings of Fatigue (POMS-B) | 4.5 ± 3.8 | 3.6 ± 3.5 | 0.24 | 4.1 ± 3.6 | 4.7 ± 4.5 | 0.17 | 0.40 (0.25 to 1.04)* | 5.4 ± 4.0 | 4.9 ± 4.7 | 0.13 | 6.4 ± 4.5 | 5.9 ± 4.0 | 0.11 | 0.00 (−0.69 to 0.69) |
P < 0.05;
significant improvement for exercise compared with quiet rest based on 95% CI not encompassing 0.
FIGURE 1—
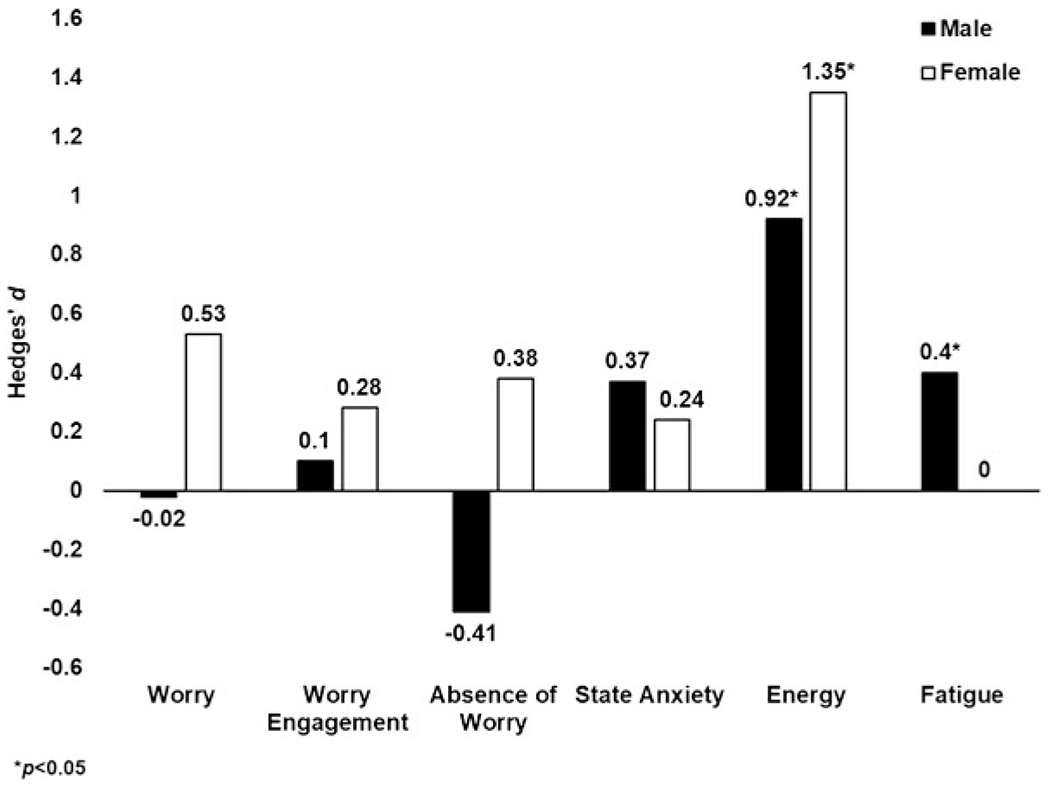
Hedges’ d Effect Sizes for Outcome Measures by Sex; asterisks indicate significant improvement for exercise compared to quiet rest based on 95% CI not encompassing 0.
Potential Moderators of Exercise Effects
Significantly larger improvements in absence of worry (t33 = 2.15, P < 0.039; d = 0.76) and state anxiety (t33 = 2.25, P ≤ 0.031; d = 0.70) were found among high-trait anxious participants. Significantly larger reductions in state anxiety were found among poor sleepers (t32 = 2.77, P ≤ 0.009; d = 0.57). No significant differences were found based on depression status or between potential moderator levels for quiet rest. Estimated energy expenditure and minutes of moderate-to-vigorous physical activity were not significantly associated with any outcome response to exercise or quiet rest.
DISCUSSION
Compared to 30 min of quiet rest, a 30-min bout of treadmill running completed at an average intensity of ~71% HRR significantly improved state anxiety and feelings of energy among young adult men and women with analogue GAD. Though not statistically significant, small magnitude improvements were found for worry, worry engagement, and feelings of fatigue. The magnitude of these improvements is consistent with previously reported responses to acute exercise among otherwise healthy adults (9,12,27), people with multiple sclerosis (28), low-income adults with elevated depressive symptoms (29), and adult women diagnosed with major depressive disorder (30). Though not significantly different, the magnitude of exercise-induced changes in worry, worry engagement, and absence of worry was larger among women than men.
Feelings of Energy
Given that low energy is a prevalent associated symptom of GAD that may contribute to negative outcomes like lower health-related quality of life (3), automobile accidents (31), and reduced achievement and productivity (32), the large, significant improvements in feelings of energy found here may be statistically and clinically meaningful. The magnitude of improvement in feelings of energy is consistent with previous reports ofmoderate-to-large improvements in feelings of energy after acute exercise and exercise training among otherwise healthy adults (9,27), young women with persistent fatigue (33), and young women with analogue (5) and clinical GAD (4). Though debates continue regarding interpretations of clinical meaningfulness, based on the suggested minimal important difference of 0.5 standard deviation (34), the 1.09 standard deviation improvement in feelings of energy here may represent a clinically meaningful finding.
Comparability of Findings for Worry and Plausible Explanations
The small magnitude improvement in worry (d = 0.22), the hallmark of GAD, found for the full sample was comparable to the previously reported effect among young adult women with analogue GAD (d = 0.25) (5). However, the magnitude of the overall effect can be attributed to a null effect among men and a moderate, but potentially clinically meaningful (34), effect among women (d = 0.53) that was twice the size of the previously reported effect (5). A poorer baseline symptom profile here may have contributed to this larger magnitude effect; the current sample of young adult women reported worse worry, state anxiety, and feelings of energy and fatigue than both the current sample of men and the previous sample with analogue GAD (5).
Consistent with a previous report that acute vigorous-intensity exercise is not sufficient to reduce worry among otherwise healthy young adult men (9), exercise trivially influenced worry and response did not differ to quiet rest. It is plausible that worry is not as sensitive to acute exercise among males, and/or that different neurobiological mechanisms underlie response and nonresponse to exercise among females and males, respectively. One plausible explanation is greater cardiac vagal tone expressed by females relative to males (35). A model of neurovisceral integration suggests that a relationship between poor vagal tone and elevated worry in GAD patients (36) may be explained by deficits in cognitive control and inhibition (37). This explanation is supported by recent evidence of an association between sex-specific attentional biases and GAD symptom profiles (38). However, because no study has examined exercise effects on autonomic function or cognitive control in GAD, how those effects influence worry or related outcome responses remains unclear.
Differential Responses to Quiet Rest
Consistent with previous findings (5,9), the magnitude of between-condition differences was influenced by differential responses to quiet rest. Hedges’ d effect sizes were larger for nearly all outcomes because of trivial or negative responses to quiet rest. It is somewhat surprising that imposed seated rest would worsen outcomes, but these findings are consistent with reported worsened mood outcomes following imposed sedentary behavior among physically active adults (39). These findings encourage future investigations of factors that may underlie worsening of mood states during imposed resting conditions (e.g., sedentary behavior and rumination) and the most appropriate comparison condition for acute exercise (e.g., minimal intervention or placebo-control conditions such as light-intensity walking or passive cycling).
Susceptibility Profiles: Plausible Role of High-trait Anxious and Poor Sleep Status
Identifying susceptibility profiles that inform the use of exercise as a precision medicine strategy is important; thus, it is notable that improvements in state anxiety and absence of worry after exercise were significantly larger among high-trait anxious participants and poor sleepers. Because trait anxiety and state anxiety are strongly correlated (14), it is unsurprising that baseline state anxiety was significantly higher among high-trait anxious participants compared to non-high-trait anxious. High state anxiety among high-trait anxious individuals and elevated worry symptoms among poor sleepers likely contributed to the larger magnitude change among these participants. Although adults with mild GAD frequently experience prolonged sleep latency, lower total sleep time, and poor sleep efficiency (40,41), no previous study has documented anxiolytic effects of acute exercise among young adults reporting poor sleep quality and analogue GAD. These findings are consistent with previous evidence that anxiety accounts for significant variability in the sleep quality of young adults (42). It is possible that postexercise symptom trajectories (43) for state anxiety and absence of worry are at least partly dependent on trait anxiety and sleep quality. Future research should leverage advanced mobile technologies (e.g., ecological momentary assessment) to probe the time-course of these effects.
Limitations
One potential limitation was the somewhat favorable baseline profile among men, characterized by lower state anxiety and feelings of fatigue and higher feelings of energy, which may not be reflective of population heterogeneity among young men with analogue or clinical GAD. Although an active lifestyle was not an exclusion criterion and physical activity was not significantly associated with outcome responses, the activity profile of included participants may be a limitation such that both exercise intensity and exercise effects may have been underestimated among highly active participants. Because physical activity is lower among other groups with elevated anxiety and/or depressive symptoms and disorders, the active group here may not accurately represent physical activity levels in analogue or clinical GAD. However, the large-scale evidence regarding physical activity among young adults with analogue or clinical GAD needed to place the current levels into context is limited, highlighting the need for future reports of physical activity levels among large samples. Using gender-based age-related norms for resting HR to estimate vigorous-intensity exercise dose has demonstrated validity (21). However, it is plausible that resting HR may be higher among high-anxious individuals and lower among the active participants here, which could have resulted in over- or underestimation of exercise intensity. Nonetheless, intensity did not significantly vary and was not associated with outcome change. Future research can benefit from larger sample sizes, comparisons of varying degrees of subclinical and clinical GAD severity, the comparison of multiple exercise modes and intensities, the use of advanced mobile technologies to elucidate the time-course of exercise response, and examination of plausible cognitive and biological mechanisms governing exercise response.
CONCLUSIONS
Notwithstanding potential limitations, these findings support the authors’ initial report of positive effects of acute aerobic exercise on worry, state anxiety, and feelings of energy and fatigue among young adult women with analogue GAD. These findings also provide initial support for these positive effects among young adult men with analogue GAD.
Acknowledgments
Brett R. Gordon is funded by the Irish Research Council under the Government of Ireland Postgraduate Programme. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
The authors disclose no conflicts of interest. The results of the present study do not constitute endorsement by the American College of Sports Medicine.
REFERENCES
- 1.Herring MP, Jacob ML, Suveg C, Dishman RK, O’Connor PJ. Feasibility of exercise training for the short-term treatment of generalized anxiety disorder: a randomized controlled trial. Psychother Psychosom. 2012;81(1):21–8. [DOI] [PubMed] [Google Scholar]
- 2.Herring MP, Kline CE, O’Connor PJ. Effects of exercise on sleep among young women with generalized anxiety disorder. Ment Health Phys Act. 2015;9:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herring MP, Johnson KE, O’Connor PJ. Exercise training and health-related quality of life in generalized anxiety disorder. Psychol Sport Exerc. 2016;27:138–41. [Google Scholar]
- 4.Herring MP, Jacob ML, Suveg C, O’Connor PJ. Effects of short-term exercise training on signs and symptoms of generalized anxiety disorder. Ment Health Phys Act. 2011;4(2):71–7. [Google Scholar]
- 5.Herring MP, Hallgren M, Campbell MJ. Acute exercise effects on worry, state anxiety, and feelings of energy and fatigue among young women with probable generalized anxiety disorder: a pilot study. Psychol Sport Exerc. 2017;33:31–6. [Google Scholar]
- 6.Wolitzky-Taylor K, Dour H, Zinbarg R, et al. Experiencing core symptoms of anxiety and unipolar mood disorders in late adolescence predicts disorder onset in early adulthood. Depress Anxiety. 2014;31(3):207–13. [DOI] [PubMed] [Google Scholar]
- 7.Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci. 2015;17(3):327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vesga-López O, Schneier F, Wang S, et al. Gender differences in generalized anxiety disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J Clin Psychiatry. 2008;69(10):1606. [PMC free article] [PubMed] [Google Scholar]
- 9.McDowell CP, Campbell MJ, Herring MP. Sex-related differences in mood responses to acute aerobic exercise. Med Sci Sports Exerc. 2016;48(9):1798–802. [DOI] [PubMed] [Google Scholar]
- 10.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28(6):487–95. [DOI] [PubMed] [Google Scholar]
- 11.Behar E, Alcaine O, Zuellig AR, Borkovec T. Screening for generalized anxiety disorder using the Penn State Worry Questionnaire: a receiver operating characteristic analysis. J Behav Ther Exp Psychiatry. 2003;34(1):25–43. [DOI] [PubMed] [Google Scholar]
- 12.Ensari I, Greenlee TA, Motl RW, Petruzzello SJ. Meta-analysis of acute exercise effects on state anxiety: an update of randomized controlled trials over the past 25 years. Depress Anxiety. 2015;32(8):624–34. [DOI] [PubMed] [Google Scholar]
- 13.Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. [DOI] [PubMed] [Google Scholar]
- 14.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the Statetrait Anxiety Inventory (Form Y): Self-evaluation Questionnaire. 1st ed. Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 15.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDSC), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–83. [DOI] [PubMed] [Google Scholar]
- 16.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 17.Fresco DM, Heimberg RG, Mennin DS, Turk CL. Confirmatory factor analysis of the Penn State Worry Questionnaire. Behav Res Ther. 2002;40(3):313–23. [DOI] [PubMed] [Google Scholar]
- 18.McNair DM, Droppleman LF, Lorr M. Edits Manual for the Profile of Mood States: POMS. Edits; 1992. [Google Scholar]
- 19.O’Connor PJ. Evaluation of four highly cited energy and fatigue mood measures. J Psychosom Res. 2004;57(5):435–41. [DOI] [PubMed] [Google Scholar]
- 20.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: US Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- 21.Miller FL, O’Connor DP, Herring MP, et al. Exercise dose, exercise adherence, and associated health outcomes in the TIGER study. Med Sci Sports Exerc. 2014;46(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–81. [PubMed] [Google Scholar]
- 23.Cook DB, O’Connor PJ, Eubanks SA, Smith JC, Lee M. Naturally occurring muscle pain during exercise: assessment and experimental evidence. Med Sci Sports Exerc. 1997;29(8):999–1012. [DOI] [PubMed] [Google Scholar]
- 24.McNeil JK, LeBlanc EM, Joyner M. The effect of exercise on depressive symptoms in the moderately depressed elderly. Psychol Aging. 1991;6(3):487–8. [DOI] [PubMed] [Google Scholar]
- 25.Cumming G The new statistics: why and how. Psychol Sci. 2013;25(1):7–29. [DOI] [PubMed] [Google Scholar]
- 26.Hedges LV, Olkin I. Statistical Methods for Meta-analysis. New York: Academic Press; 1985. [Google Scholar]
- 27.Loy BD, O’Connor PJ, Dishman RK. The effect of a single bout of exercise on energy and fatigue states: a systematic review and meta-analysis. Fatigue. 2013;1(4):223–42. [Google Scholar]
- 28.Ensari I, Sandroff BM, Motl RW. Intensity of treadmill walking exercise on acute mood symptoms in persons with multiple sclerosis. Anxiety Stress Coping. 2017;30(1):15–25. [DOI] [PubMed] [Google Scholar]
- 29.Legrand FD, Race M, Herring MP. Acute effects of outdoor and indoor exercise on feelings of energy and fatigue in people with depressive symptoms. J Environ Psychol. 2018;56:91–6. [Google Scholar]
- 30.Meyer JD, Koltyn KF, Stegner AJ, Kim J-S, Cook DB. Influence of exercise intensity for improving depressed mood in depression: a dose-response study. Behav Ther. 2016;47(4):527–37. [DOI] [PubMed] [Google Scholar]
- 31.Martiniuk AL, Senserrick T, Lo S, et al. Sleep-deprived young drivers and the risk for crash: the DRIVE prospective cohort study. JAMA Pediatr. 2013;167(7):647–55. [DOI] [PubMed] [Google Scholar]
- 32.Fukuda S, Yamano E, Joudoi T, et al. Effort-reward imbalance for learning is associated with fatigue in school children. Behav Med. 2010;36(2):53–62. [DOI] [PubMed] [Google Scholar]
- 33.Herring MP, O’Connor PJ. The effect of acute resistance exercise on feelings of energy and fatigue. J Sports Sci. 2009;27(7):701–9. [DOI] [PubMed] [Google Scholar]
- 34.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;41(5):582–92. [DOI] [PubMed] [Google Scholar]
- 35.Abhishekh HA, Nisarga P, Kisan R, et al. Influence of age and gender on autonomic regulation of heart. J Clin Monit. 2013;27(3):259–64. [DOI] [PubMed] [Google Scholar]
- 36.Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biol Psychiatry. 1996;39(4):255–66. [DOI] [PubMed] [Google Scholar]
- 37.Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33(2):81–8. [DOI] [PubMed] [Google Scholar]
- 38.Kinney KL, Boffa JW, Amir N. Gender difference in attentional bias toward negative and positive stimuli in generalized anxiety disorder. Behav Ther. 2017;48(3):277–84. [DOI] [PubMed] [Google Scholar]
- 39.Edwards MK, Loprinzi PD. Experimentally increasing sedentary behavior results in increased anxiety in an active young adult population. J Affect Disord. 2016;204:166–73. [DOI] [PubMed] [Google Scholar]
- 40.Monti JM, Monti D. Sleep disturbance in generalized anxiety disorder and its treatment. Sleep Med Rev. 2000;4(3):263–76. [DOI] [PubMed] [Google Scholar]
- 41.Papadimitriou GN, Linkowski P. Sleep disturbance in anxiety disorders. Int Rev Psychiatry. 2005;17(4):229–36. [DOI] [PubMed] [Google Scholar]
- 42.Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46(2):124–32. [DOI] [PubMed] [Google Scholar]
- 43.Dyer JB III, Crouch JG. Effects of running on moods: a time series study. Percept Mot Skills. 1987;64(3):783–9. [Google Scholar]


