Table 4.
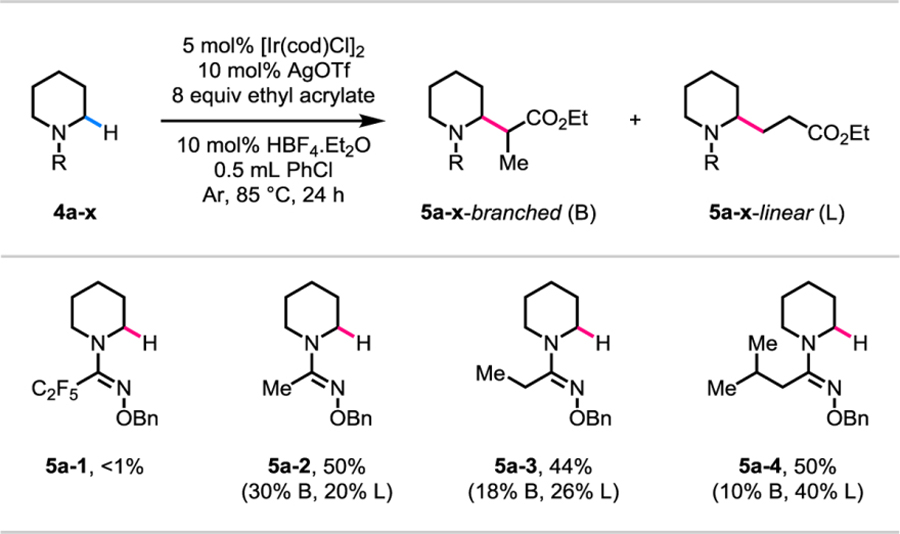 |
Reaction conditions: 4a-1 to 4a-4 (0.1 mmol, 1.0 equiv), [Ir(cod)Cl]2 (0.005 mmol, 0.05 equiv), AgOTf (0.01 mmol, 0.1 equiv), ethyl acrylate (0.8 mmol, 8.0 equiv), HBF4.Et2O (0.01 mmol, 0.1 equiv), degassed PhCl (0.5 mL), 85 °C, under Ar, 24 h.
Yields were determined by 1H NMR analysis of the crude products using mesitylene as the internal standard.
