Figure 1. The structural arrangement of Hsp70 molecular chaperones.
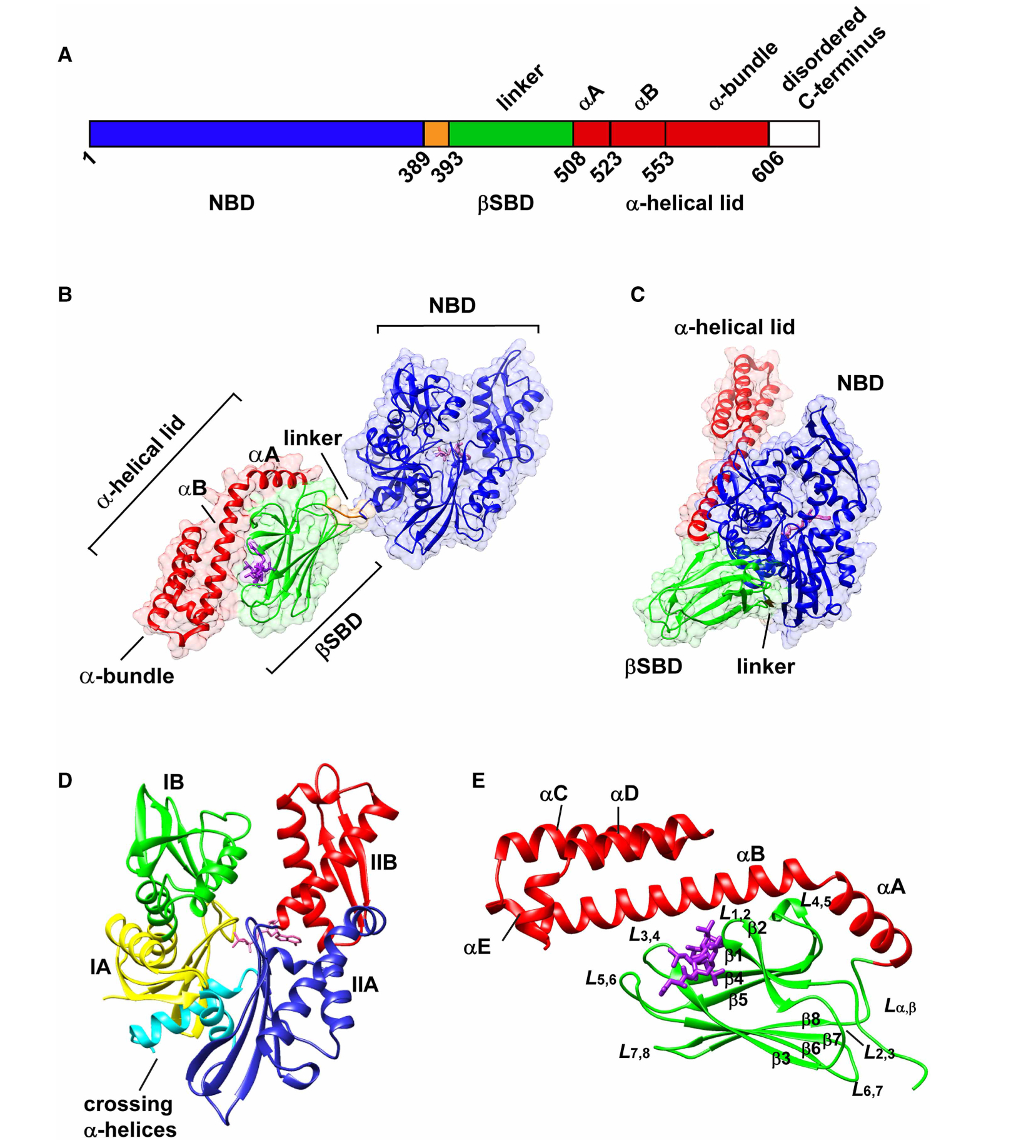
(A) Schematic representation of the structural domains of Hsp70s. The sequence numbers are based on the E. coli Hsp70, DnaK. Structures of DnaK showing (B) the canonical ADP-bound undocked state (PDB ID 2kho [12]), and (C) the canonical ATP-bound docked state (PDB ID 4b9q [17]). The NBD is colored in blue. In the substrate-binding domain (SBD), the βSBD is colored green, α-lid in red, and the linker in orange. The bound nucleotide and the peptide substrate NRLLLTG are shown in purple. (D) Subdomain organization of the NBD of a representative Hsp70. The structure shown is ADP-bound Hsc70 (PDB ID 3hsc [92]). Subdomains IA, IB, IIA, and IIB are colored in yellow, green, blue, and red, respectively. The crossing α-helices are shown in cyan. (E) Structure of the DnaK SBD bound to the peptide NRLLLTG (PDB 1dkz [39]). The binding mode of peptide substrate is illustrated in Figure 7.
