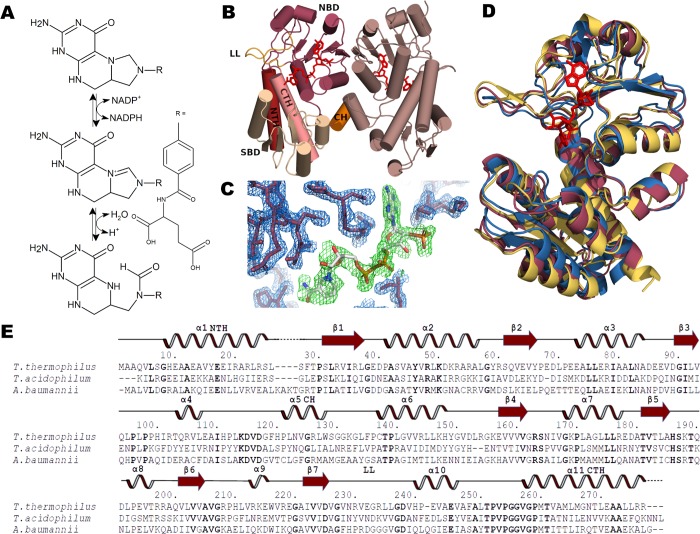
Fig 1. MTHFD structure and function.
(A) MTHFD catalyzes the conversion of 5,10‐methylenetetrahydrofolate via 5,10-methenyltetrahydrofolate to 10-formyltetrahydrofolate in a NADP+ dependent manner. (B) Secondary structural elements of the T. thermophilus MTHFD dimer. Important structural elements are indicated. LL: lid loop, NTH: N-terminal helix, SBD: substrate binding domain, CH: connector helix, NBD: nucleotide binding domain, CTH: C-terminal helix. (C) 2Fo-Fc electron density map at the 1.0 σ level of the active site of T. thermophilus MTHFD showing the NADP+ nucleotide. (D) Alignment of Thermus thermophilus (red) Thermoplasma acidophilum (yellow) Acinetobacter baumannii (blue) crystal structures. (E) Amino acid sequence alignment of MTHFD from T. thermophilus, T. acidophilum and A. baumannii with secondary structure elements (α-helices, β-strands as arrows) of T. thermophilus indicated above sequences. Numbering (indicated by dots) refers to the T. thermophilus sequence.