Abstract
To report on the characteristics and outcome of management of Coats’ disease, and to describe a novel surgical technique for management of stage 3B with total retinal detachment (RD) by scleral external drainage with anterior chamber (AC-maintainer) placement before the drainage without pars plana vitrectomy.
A retrospective study of 26 eyes from 25 Coats’ patients. Outcome measures included: demographics, presentation, laterality, stage, treatment, and outcome.
The median age at diagnosis was 3.5 years. Twenty patients (80%) were males, and all except 1 girl had unilateral disease. The presenting complaint was impaired vision in 13 (50%) eyes, leukocoria in 6 (23%) eyes, and strabismus in 7 (27%) eyes. Based on the Shields classification; 3(12%) eyes were stage 1, 9 (35%) eyes were stage 2, 10 (38%) eyes were stage 3, 2 (8%) eyes were stag e4, and 2 (8%) eyes were stage 5. Primary management included cryotherapy (54%), laser photocoagulation (27%), intravitreal anti-vascular endothelial growth factor (23%), intravitreal steroids (23%), and surgical drainage (12%). The 3 eyes in stage 3B (with total exudative RD) underwent subretinal fluid drainage with AC maintainer, and all had the retina reattached completely for 6 months follow up after the surgery. At mean follow up 21 months, 4 (15%) eyes were enucleated, 19 (73%) eyes had improvement or stabilization in visual acuity.
Coats’ disease usually presents at advanced stage with poor visual prognosis, individualized management with close follow up are mandatory to save the eye. Subretinal fluid drainage with AC maintainer is a safe and useful technique for repairing total RD in eyes with stage 3B Coats’ disease.
Keywords: coats disease, cryotherapy, leukocoria, retinoblastoma
1. Introduction
Coats’ disease was defined as idiopathic retinal telangiectasia with intra-retinal and/or sub-retinal exudation, which can lead to exudative retinal detachment (RD) in absence of vitreo-retinal traction. It is a rare progressive disorder that is usually unilateral, and affecting mainly young males.[1,2]
This condition was originally described by Coats in 1908, though his series also included eyes with retinal capillary hemangiomas (Von-Hippel Lindau disease).[3] In 1956, Reese refined the definition and description into the condition we would now recognize as Coats’ disease.[4] Coats patients usually present with decreased visual acuity, strabismus, or leukocoria, and less commonly they present with eye pain, heterochromia of the iris, nystagmus. Rarely it can be asymptomatic in early stages and diagnosed during routine ophthalmologic examination.[1,2]
Even early diagnosed cases can be stabilized with absorption of the exudation, more advanced cases may progress to complete RD, neovascular glaucoma, and sometimes may require enucleation.[1,2] Several approaches were described in the management of Coats’ disease; including pars plana vitrectomy (PPV) surgery, cryotherapy, laser photocoagulation and intravitreal steroids and/or anti-vascular endothelial growth factor (VEGF) injections.[5,6]Figure 1 shows an example of a case with stage 2 Coats managed by laser photocoagulation targeted to the ischemic retina as detected by fundus fluorescein angiography with subsequent resolution of retinal exudation.
Figure 1.
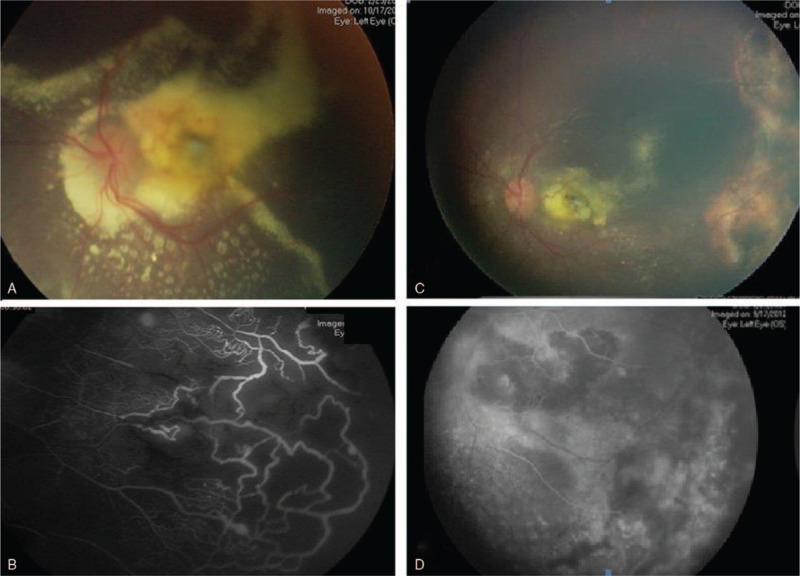
(A) The left eye had extensive subretinal exudates associated with peripheral telangiectasia and ischemia as detected in FFA (B). After treatment by laser photocoagulation for the peripheral ischemic retina the eye has less exudates (C), and ablated ischemic area (D). FFA = fundus fluorescein angiography.
Based on Shields classification,[7] Coats’ disease can be staged as follow: retinal telangiectasia (stage 1) as shown in Figure 2, retinal telangiectasia plus extra-foveal and foveal exudation (stage 2a and 2b), subtotal and total exudative RD (stages 3a and 3b), RD plus secondary glaucoma (stage 4), and advanced end-stage (stage 5).[1,7]
Figure 2.
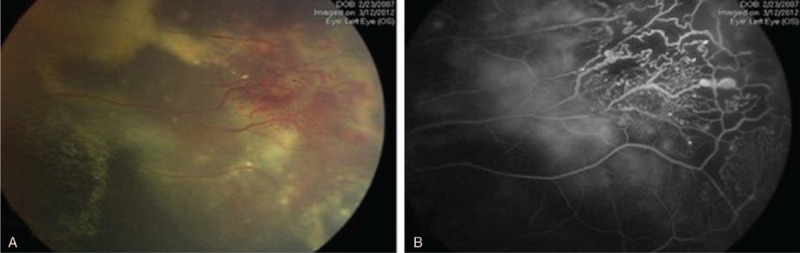
(A) Fundus photo showed the characteristic telangiectasia in Coats’ disease, which is associated with peripheral retinal ischemia as detected in fundus fluorescein angiography (FFA) (B).
Eyes present with stage 3B coats disease have total exudative RD, and there is no uniform management option for this stage. Available options include, PPV with silicon oil insertion, and scleral external drainage. As Coats’ disease is a progressive talengectic disease with leaky vessels, PPV may not solve the issue on the long term follow up, and external scleral drainage alone may lead to hypotony and its associated complications.
Herein, we analyzed the presentation and management for patients with Coats’ disease in a specialized center in Jordan, and we described the outcome of use of a novel surgical technique for management of stage 3B eyes by external drainage with anterior chamber (AC)-maintainer. This experience should help ophthalmologists in understanding the behavior of this serious eye and vision threatening disease, and should allow them to plan treatment for patients with Coats’ disease at different stages case by case.
2. Methods
The study was approved by the Institutional Review Board. It is a retrospective case series of 26 eyes from 25 consecutive patients from January 2011 to December 2017 who had a clinical diagnosis of Coats’ disease at King Hussein Cancer Center (Amman, Jordan), and in Ibn Al-Haitham Hospital (Amman, Jordan). The diagnosis was established on the basis of clinical features including retinal telangiectasia, exudation, RD, and the absence of other vitreoretinal diseases, such as retinoblastoma. All cases were evaluated by vitreoretinal surgeon and ocular oncologist.
Each patient underwent a complete ophthalmic examination (examination under general anesthesia was performed for very young patients) that included indirect ophthalmoscopy, fluorescein angiography, ultrasound B-scan, and magnetic resonance imaging if needed. Fundus photos were taken for documentation, and each eye was retrospectively re-staged based on Shields classification for Coats’ disease.[7] Selection required access to patients’ medical, radiological, and pathologic records. Data collected included patient's age, gender, laterality, age at diagnosis, disease stage, modalities of treatment, follow up, outcomes, and best-corrected visual acuity at the first and last examination.
2.1. Inclusion and exclusion criteria
The eligibility criteria for inclusion were eyes with clinical diagnosis of Coats’ disease. Eyes that had clinical features of other vitreoretinal diseases, intraocular tumors like retinoblastoma, and persistent hyperplastic primary vitreous were excluded. Patients who refused treatment or were followed for less than 6 months were not included in management outcome.
2.2. Surgical technique
External Drainage with AC-Maintainer was done for eyes with Coats’ disease stage 3B. The technique involved placement of the anterior chamber maintainer (ACM) followed by limbal conjunctival peritomy incision and hocking of the recti muscles to allow visualization of the sclera, then a radial sclerotomy about 1 to 2 mm in length was placed posterior to the equator.
A 7-0 Vicryl suture was placed to secure the closure of sclerotomy after the subretinal fluid drainage, and then ACM was opened. The subretinal fluid was drained vie the sclerotomy using a 27-gauge needle. The sclerotomy has to be enlarged to allow the oily and exudative materials to be drained, with the ACM the globe stability was preserved throughout the whole procedure, the balanced salt solution entered the vitreous chamber through the lens zonules and the retina was completely attached and dry at the end of the procedure.
3. Results
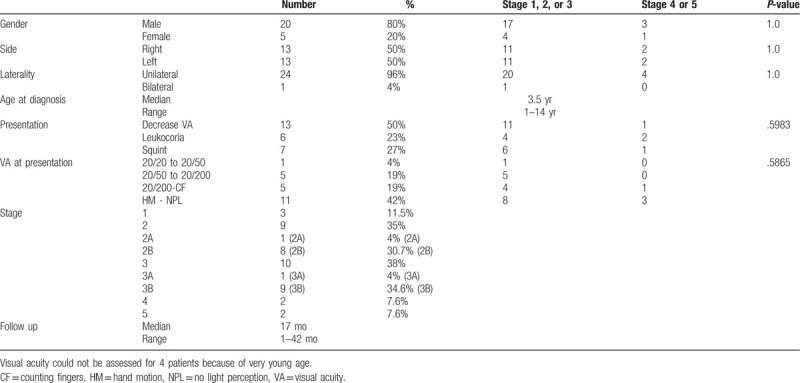
The mean age at diagnosis was 10 years (range; 1–14 years). Twenty patients (80%) were males, 5 (20%) were females, and all except 1 girl had unilateral disease. The presenting complaint was decreased visual acuity in 13 (50%) eyes, leukocoria in 6 (23%) eyes, and strabismus in 7 (27%) eyes (Table 1). Snellen visual acuity was 20/20 to 20/50 in 1 eye (4%), 20/50 to 20/200 in 5 eyes (19%), 20/200-counting fingers in 5 eyes (19%), and hand motion to no light perception in 11 eyes (42%). Based on the Shields classification; 3(12%) eyes were stage 1, 9 (35%) eyes were stage 2, and 10 (38%) eyes were stage 3, 2 (8%) eyes were stage 4, and 2 (8%) were stage 5 (Table 1).
Table 1.
Demographics and disease characteristics.
3.1. Treatment modalities and outcome
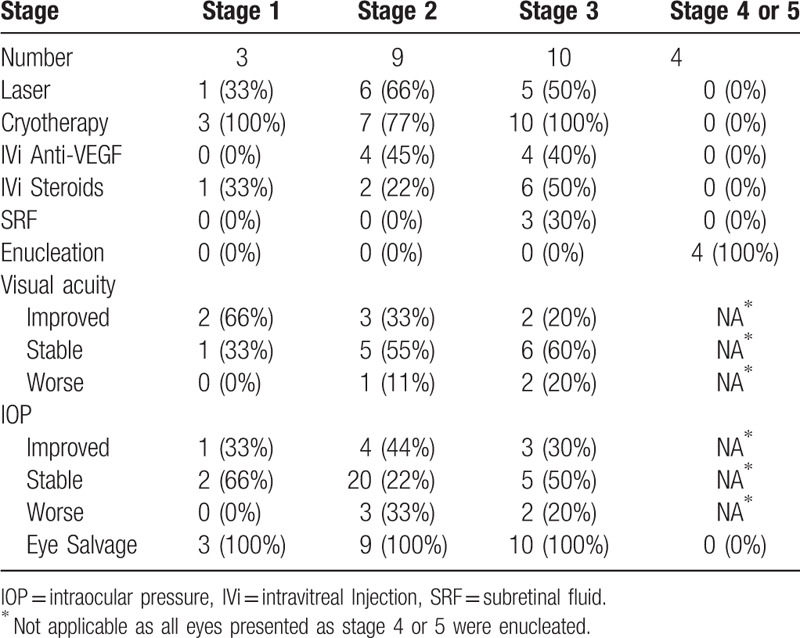
Primary management included cryotherapy (54%), laser photocoagulation (27%), intravitreal Anti VEGF (23%), intravitreal steroids (23%), and surgical drainage (12%). Ten (38%) eyes required multiple sessions to stabilize the disease, and 4 (15%) eyes were enucleated because of clinical suspicion of retinoblastoma or the presence of end stage glaucoma (Table 2). Three eyes in stage 3B (with total exudative RD) underwent subretinal fluid drainage with AC maintainer (combined with laser photocoagulation and cryotherapy), and all had the retina reattached completely after surgery. At mean follow up 21 months (range; 15–42 months), 4 (15%) eyes were enucleated, 6 (23%) eyes had significant improvement in visual acuity, 13 (50%) eyes had stable visual acuity, and all (100%) non enucleated eyes had controlled intraocular pressure (IOP) (Table 2).
Table 2.
Management modalities and outcomes for 26 eyes with Coats’ disease.
3.2. Eyes treated by scleral external drainage with AC-maintainer
3.2.1. Case 1
A 2-year-old girl presented with right eye leukocoria and left eye exotropia. Her fundus revealed macular exudates (Stage 2B) in the right eye and total exudative RD (Stage 3B) in the left eye. The IOP was 15 mm Hg in both eyes. She was diagnosed to have bilateral Coats’ disease and underwent cryotherapy for the right eye, and subretinal fluid drainage with ACM, cryotherapy, laser photocoagulation, and intravitreal triamcinolone injection for the left eye. At 6 months follow up, she had flat retina in the left eye and IOP was 13 mm Hg as well as stable disease in the right eye with flat retina and IOP was 11 mm Hg.
3.2.2. Case 2
A 3.5-year-old boy presented with right leukocoria, examination showed total RD with subretinal yellowish fluid (IOP was 19 mm Hg) in the right eye and he was diagnosed to have stage 3B Coats’ disease. He underwent subretinal fluid drainage with ACM with cryotherapy and intravitreal anti-VEGF injection. At 2 months follow up the fundus exam showed a flat retina with IOP of 15 mm Hg with total absorption of the exudates.
3.2.3. Case 3
A 1.5-year-old girl presented with right eye exotropia, and found to have total RD with massive subretinal (yellowish) deposits (stage 3B coats disease) in her right eye and the IOP was 17 mm Hg with normal left eye. She underwent subretinal fluid drainage with ACM with cryotherapy and intravitreal triamcinolone injection (Fig. 3). At 6 months follow up she had a flat retina with minimal exudates and the telangiectatic vessels were treated by laser photocoagulation, and the IOP was 12 mm Hg. At 15 months follow up she developed cataract which was treated by a lensectomy with anterior vitrectomy. The IOP remained 14 mm Hg in the affected eye and the retina remained flat with stable disease.
Figure 3.
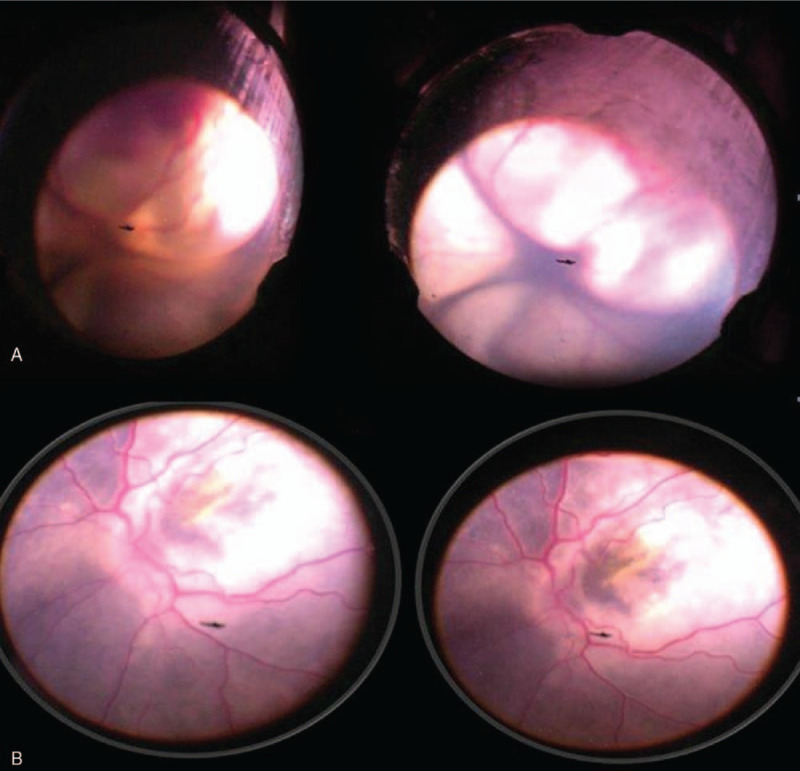
(A) 1.5-yr-old girl who had stage 3b Coats’ disease (total exudative retinal detachment) (case 3). She underwent subretinal fluid drainage with anterior chamber maintainer and cryotherapy. The retina was flat by the end of the procedure (B).
4. Discussion
The incidence of Coats’ disease is 0.09 per 100,000 population,[8,9] and although it is uncommon disease, it is well known to most ophthalmologists and has been the subject of several small and large case series.[1,10–14] As in other reports, 96% of cases in our series were unilateral, with predominance for young males (80%).[1,7,15–17] Two large retrospective studies from the United States and India and 1 prospective study from the United Kingdom have been published, and addressed the demographics, clinical features, treatment modalities, and outcomes of the disease.[1,7,15,16] Data from the middle east is missed in the literature except a study from Saudi Arabia,[17] and herein we study Coats disease in the Jordanian population.
Shields reported a median age at presentation of 5 years,[1] while in United Kingdom it was 8 years.[18] The younger age of presentation in shields group was explained by the fact that many younger children were referred to the ocular oncology service at the Wills Eye Hospital because of concern about possible underlying retinoblastoma by the referring doctor.[1] Similarly, in a different series of 10 children with Coats’ disease reported by Char,[14] and who were originally referred because of suspected retinoblastoma, the mean age at diagnosis was 2.4 years. In our series, the median age at diagnosis was 3.5 years, and this young age also could be expanded because our institute (King Hussein Cancer Center, Amman, Jordan) is a tertiary cancer center where many cases were younger patients who were referred as suspicious cases of Retinoblastoma. The main clinical findings in our series were similar to those reported in previous studies.[8,1,7,15–19] The hallmark of the disease, retinal telangiectasia, was found in all eyes when fundus view was possible. Shields et al[7]-as in our series- reported that the most common presentation was Stage 3B disease, an advanced stage characterized by total exudative RD without high IOP. This may be a referral bias knowing that most if not all cases reported by Shields et al were presumably seen in a major ocular oncology center where more advanced cases are likely to be referred because of concerns of retinoblastoma which is exactly the case in our institution.[1,7]
Coats’ disease is associated with a very poor vision (LogMAR vision ≤1.0) at presentation in a large proportion of cases (44%–88%) in all published case series including this report.[1,10,12,15] On the other hand leukocoria is the presenting sign for Coats disease in 20% to 24% of cases,[1,15] and in 23% of cases in this report; hence, it is an important consideration in the differential diagnosis of retinoblastoma.[18,20] In our series, 2 eyes were enucleated for the suspicion of retinoblastoma. And histopathology for the enucleated specimens showed features consistent with Coats disease rather than retinoblastoma as shown in Figure 4. The history in a patient with leukocoria is critical. The age of onset, the perinatal history, and the family history as well as the clinical examination and the color of exudation are important variables in differentiating Coats’ disease from other common causes of leukocoria, particularly retinoblastoma and retinopathy of prematurity.
Figure 4.
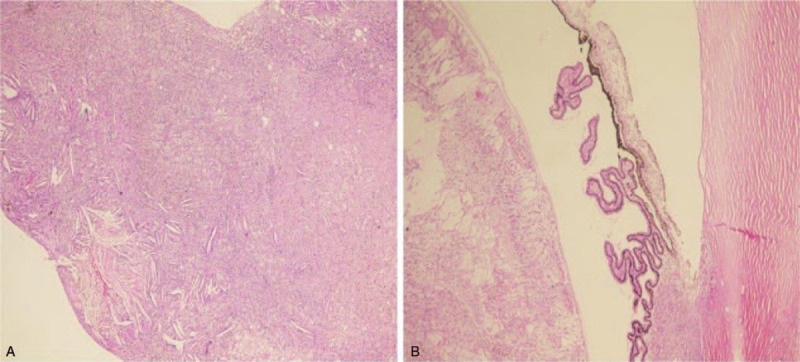
Histopathology in advanced Coats’ disease. (A) Photomicrograph showing typical proteinaceous SRF with cholesterol clefts in an eye enucleated for advanced Coats’ disease (Original magnification ·100, hematoxylin and eosin). (B) Photomicrograph of anterior chamber and the ciliary body showing cholesterol clefts (Original magnification ·100, hematoxylin and eosin), and there were no tumor cells suggestive of Retinoblastoma. SRF = subretinal fluid.
Proper diagnosis and tailored management to avoid unfavorable complications like blindness, painful glaucoma, and enucleation are required. Treatment modalities include laser therapy, cryotherapy, intravitreal steroids, intravitreal anti-VEGF, and sub retinal fluid drainage. Eventhough there is no uniformed protocol for the management of all cases. Multiple factors should be considered before choosing the proper method for any given patient, varying from current retinal status and the presence of complications at the time of diagnosis, the cost, and the availability for follow up. In our series, most of cases mandated more than 1 treatment, and visual acuity improved with treatment in 1 quadrant of the cases.[1,21–24] For patients with minimal peripheral ischemia and no visual impairment, observation can be an option, while in patients with peripheral retinal telangiectasia with little or no exudative detachment, photocoagulation for the ischemic retina may be sufficient.[5–7] When laser photocoagulation is not possible, even being the treatment of choice for mild Coats’ disease, cryotherapy could be more effective.[1,21–24] Cryotherapy was the most common modality of treatment used in our series, similar to Shields et al report,[1,7] but unlike the Saudi experience were laser photocoagulation was the most commonly used therapy.[17] The majority of the eyes required further treatment sessions after initial treatment in all reports. This emphasizes the importance of long-term follow-up and the potential need for multiple treatment sessions. Anti-VEGF and intravitreal steroids have been used in the treatment of Coats’ disease,[21–25] as it seems to reduce macular edema and exudates, and enhance the regression of dilated abnormal vessels.[23,25,26] Data about safety of anti-VEGF in this group is limited,[27] while intravitreal steroids harbor a more predictable systemic side effects among pediatricians. That's why we were more convenient with the use of steroids in this series, eventhough increased IOP as well as the risk of cataract remained major concerns, and closer follow ups were required. In more advanced cases where vitreoretinal surgery is indicated, the choice to do external subretinal fluid drainage which seems to accelerate the flattening of retina that, theoretically, increases the chances of improvement in visual acuity,[28] but it could lead to hypotony.[28] Therefore we did a modification in this technique in 3 cases of stage 3b coats disease by placing ACM, therefore the fluctuations of intraoperative IOP were minimized and the response was favorable. Despite adequate ablative therapy and obliteration of the telangiectasias in many cases, restoration of vision is difficult. More than half of the eyes in this series had 20/200 or worse vision at last follow-up, that is similar to reports from the United States (64%), the United Kingdom (51%), and Saudi Arabia (59%).[1,15,17] And unfortunately enucleation may be indicated when there is severe complications of the disease. In our series, almost 15% of the eyes were eventually enucleated, which is similar to previous reports in the literature. The 2 main reasons for enucleation were suspicion of retinoblastoma and neovascular glaucoma.[7,16,17]
In conclusion, Coats’ disease in Jordanian population has similar clinical characteristics and treatment outcomes as compared to other ethnic populations. This finding suggests that Coats disease manifests similarly across varied populations. Appropriate diagnosis and therapeutic approach are mandatory in the management to ensure a relatively good visual acuity. A tailored and individualized management for every case with close follow up remains the best approach for most of our cases. We acknowledge our study has limitations. It is retrospective and of limited size. Despite these limitations, we believe our study remains pertinent to the management of coats disease. A larger and more comprehensive multicenter and international study should be performed to better analyze the management options and prognosis for each stage of coats disease.
Author contributions
Conceptualization: Yacoub A. Yousef, Mona Mohammad.
Data curation: Yacoub A. Yousef, Mona Mohammad, Omar Abureesh.
Formal analysis: Yacoub A. Yousef.
Funding acquisition: Yacoub A. Yousef and Mario Damiano Toro.
Investigation: Yacoub A. Yousef, Ahmad H. ElRimawi, Ahmad F. Qaroot.
Methodology: Yacoub A. Yousef, Mona Mohammad, Omar Abureesh, Katarzyna Nowomiejska.
Project administration: Yacoub A. Yousef, Mario Damiano Toro, Ibrahim AlNawaiseh.
Resources: Yacoub A. Yousef.
Software: Yacoub A. Yousef.
Supervision: Yacoub A. Yousef, Adnan H. AlAref, Robert Rejdak, Teresio Avitabile.
Validation: Yacoub A. Yousef, Mona Mohammad, Teresio Avitabile.
Visualization: Yacoub A. Yousef.
Writing – original draft: Yacoub A. Yousef.
Writing – review & editing: Yacoub A. Yousef, Mona Mohammad, Omar Abureesh, Mario Damiano Toro, Ibrahim AlNawaiseh.
Mario Damiano Toro orcid: 0000-0001-7152-2613.
Footnotes
Abbreviations: AC = anterior chamber, ACM = anterior chamber maintainer, IOP = intraocular pressure, PPV = pars plana vitrectomy, RD = retinal detachment, VEGF = vascular endothelial growth factor.
How to cite this article: Yousef YA, ElRimawi AH, Nazzal RM, Qaroot AF, AlAref AH, Mohammad M, Abureesh O, Rejdak R, Nowomiejska K, Avitabile T, Toro MD, AlNawaiseh I. Coats’ disease: characteristics, management, outcome, and scleral external drainage with anterior chamber maintainer for stage 3b disease. Medicine. 2020;99:16(e19623).
This study was supported by the “Foundation to support the development of Ophthalmology”, Lublin, Poland.
The authors have no conflicts of interest to disclose.
References
- [1].Shields JA, Shields CL, Honavar SG, et al. Clinical variations and complications of Coats disease in 150 cases: the 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol 2001;131:561–71. [DOI] [PubMed] [Google Scholar]
- [2].Spitznas M, Joussen F, Wessing A, et al. An epidemiologic and Fluorescein angiographic study. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1975;195:241–50. [DOI] [PubMed] [Google Scholar]
- [3].Coats G. Forms of retinal diseases with massive exudation. Roy Lond Ophthalmol Hosp Rep 1908;17:440–525. [Google Scholar]
- [4].Reese AB. Telangiectasis of the retina and Coats’ disease. Am J Ophthalmol 1956;42:1–8. [DOI] [PubMed] [Google Scholar]
- [5].Ridley ME, Shields JA, Brown GC, et al. Coats’ disease. Evaluation of management. Ophthalmology 1982;89:1381–7. [DOI] [PubMed] [Google Scholar]
- [6].Jun JH, Kim YC, Kim KS. Resolution of severe macular edema in adult coats’ disease with intravitreal triamcinolone and bevacizumab injection. Korean J Ophthalmol 2008;22:190–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shields JA, Shields CL, Honavar SG, et al. Classification and management of Coats disease: the 2000 Proctor lecture. Am J Ophthalmol 2001;131:572–83. [DOI] [PubMed] [Google Scholar]
- [8].Shields JA, Shields CL. Review: coats disease: the 2001 LuEsther T. Mertz lecture. Retina 2002;22:80–91. [DOI] [PubMed] [Google Scholar]
- [9].Jones JH, Kroll AJ, Lou PL, et al. Coats’ disease. Int Ophthalmol Clin 2001;41:189–98. [DOI] [PubMed] [Google Scholar]
- [10].Cahill M, O’Keefe M, Acheson R, et al. Classification of the spectrum of Coats’ disease as subtypes of idiopathic retinal telangiectasis with exudation. Acta Ophthalmol Scand 2001;79:596–602. [DOI] [PubMed] [Google Scholar]
- [11].Foot B, Stanford M, Rahi J, et al. British Ophthalmological Surveillance Unit Steering Committee. The British Ophthalmological Surveillance Unit: an evaluation of the first 3 years. Eye (Lond) 2003;17:9–15. [DOI] [PubMed] [Google Scholar]
- [12].Budning AS, Heon E, Gallie BL. Visual prognosis of Coats’ disease. J AAPOS 1998;2:356–9. [DOI] [PubMed] [Google Scholar]
- [13].Smithen LM, Brown GC, Brucker AJ, et al. Coats’ disease diagnosed in adulthood. Ophthalmology 2005;112:1072–8. [DOI] [PubMed] [Google Scholar]
- [14].Char DH. Coats’ syndrome: long term follow-up. Br J Ophthalmol 2000;84:37–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Morris B, Foot B, Mulvihill A. A population-based study of Coats disease in the United Kingdom I: epidemiology and clinical features at diagnosis. Eye (Lond) 2010;24:1797–801. [DOI] [PubMed] [Google Scholar]
- [16].Rishi P, Rishi E, Uparkar M, et al. Coats’ disease: an Indian perspective. Indian J Ophthalmol 2010;58:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Al-Qahtani AA, Almasaud JM, Ghazi NG. Clinical characteristics and treatment outcomes of coats disease in a saudi arabian population. Retina 2015;35:2091–9. [DOI] [PubMed] [Google Scholar]
- [18].Shields JA, Parsons HM, Shields CL, et al. Lesions simulating retinoblastoma. J Pediatr Ophthalmol Strabismus 1991;28:338–40. [DOI] [PubMed] [Google Scholar]
- [19].Haik BG. Advanced Coats’ disease. Trans Am Ophthalmol Soc 1991;89:371–476. [PMC free article] [PubMed] [Google Scholar]
- [20].Shields CL, Schoenberg E, Kocher K, et al. Lesions simulating retinoblastoma (pseudoretinoblastoma) in 604 cases: results based on age at presentation. Ophthalmology 2013;120:311–6. [DOI] [PubMed] [Google Scholar]
- [21].Nucci P, Bandello F, Serafino M, et al. Selective photocoagulation in Coats’disease: ten-year follow up. Eur J Ophthalmol 2002;12:501–5. [PubMed] [Google Scholar]
- [22].Theodossiadis GP. Some clinical, fluorescein-angiographic, and therapeutic-aspects of Coats’ disease. J Pediatr Ophthalmol Strabismus 1979;16:257–62. [DOI] [PubMed] [Google Scholar]
- [23].Ozdamar Y, Berker N, Batman C, et al. Vitreoretinal surgery in advanced Coats disease. Retinal Cases Brief Rep 2009;3:57–9. [DOI] [PubMed] [Google Scholar]
- [24].Ghorbanian S, Jaulim A, Chatziralli IP. Diagnosis and treatment of coats’ disease: a review of the literature. Ophthalmologica 2012;227:175–82. [DOI] [PubMed] [Google Scholar]
- [25].Jonas J. Intravitreal triamcinolone acetonide as treatment forextonsive exudative retinal detachment. Br J Ophthalmol 2004;88:587–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kaul S, Uparkar M, Mody K, et al. Intravitreal anti-vascular endothelial growth factor agents as anadjunct in the management of Coats’ disease in children. Indian J Ophthalmol 2010;58:76–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pertl L, Steinwender G, Mayer C, et al. A systematic review and meta-analysis on the safety of vascular endothelial growth factor (VEGF) inhibitors for the treatment of retinopathy of prematurity. PLoS One 2015;10:e0129383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Imaizumi A, Kusaka S, Takaesu S, et al. Subretinal fluid drainage and vitrectomy are helpful in diagnosing and treating eyes with advanced Coats’ disease. Case Rep Ophthalmol 2016;7:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


