Abstract
Background:
In recent years, metastatic castration-resistant prostate cancer (MCRPC) and studies related to MCRPC have drawn global attention. The main objective of this bibliometric study was to provide an overview of MCRPC, explore clusters and trends in research and investigate the future direction of MCRPC research.
Methods:
A total of 4089 publications published between 1979 and 2018 were retrieved from the Web of Science (WoS) Core Collection database. Different aspects of MCRPC research, including the countries/territories, institutions, journals, authors, research areas, funding agencies and author keywords, were analyzed.
Results:
The number of annual MCRPC publications increased rapidly after 2010. American researchers played a vital role in this increase, as they published the most publications. The most productive institution was Memorial Sloan Kettering Cancer Center. De Bono, JS (the United Kingdom [UK]) and Scher, HI (the United States of America [USA]) were the two most productive authors. The National Institutes of Health (NIH) funded the largest number of published papers. Analyses of keywords suggested that therapies (abiraterone, enzalutamide, etc.) would attract global attention after US Food and Drug Administration (FDA) approval.
Conclusions:
Developed countries, especially the USA, were the leading nations for MCRPC research because of their abundant funding and frequent international collaborations. Therapy was one of the most vital aspects of MCRPC research. Therapies targeting DNA repair or the androgen receptor (AR) signing pathway and new therapies especially prostate-specific membrane antigen (PSMA)-based radioligand therapy (RLT) would be the next focus of MCRPC research.
Keywords: bibliometric analysis, bone metastases, drug resistance, metastatic castration-resistant prostate cancer (MCRPC), prostate-specific membrane antigen (PSMA), therapy
1. Introduction
Prostate cancer (PCa) is one of the most prevalent malignancies in the world1,2,3 and the third most common cause of male cancer-related death in the United States of America (USA).[1] The majority of men with newly diagnosed PCa present with localized disease and undergo radical prostatectomy and/or radiological therapy, followed by androgen deprivation therapy (ADT).4,5 Depending on the grade of the cancer, a variable percentage of these patients experience progression to castration-resistant prostate cancer (CRPC) within 10 years.6,7 CRPC was previously named “hormone-refractory prostate cancer” and “androgen-independent prostate cancer”.8,9 However, because castration treatments including ADT were ineffective, these cancers still showed reliance upon hormones for androgen receptor (AR) activation.[10] Thus, “hormone-refractory prostate cancer” and “androgen-independent prostate cancer” were replaced by the term “castration-resistant prostate cancer (CRPC)”.[11] Although metastatic castration-resistant prostate cancer (MCRPC) patients currently benefit from a wealth of effective treatment options, MCRPC remains incurable, and the prognosis of these patients is quite poor. For men with CRPC, the median survival ranges from 9 to 30 months, and for those with MCRPC, this survival is reduced to 9–13 months.[12]
Currently, cytotoxic chemotherapy agents, AR blocking agents, immunotherapies, and radiopharmaceuticals represent effective therapeutic strategies for MCRPC treatment.7,13 Taxane chemotherapy (docetaxel and cabazitaxel) is the standard for MCRPC treatment.7,14,15 Abiraterone and enzalutamide represent significant breakthroughs in the treatment of MCRPC and bestow significant survival benefits.16,17,18,19,20,21,22,23 Sipuleucel-T, a novel active cellular immunotherapy, can prolong the overall survival (OS) of men with MCRPC.13,24,25,26,27 Radium-223 (Ra 223) dichloride targets bone metastases with high-energy, short-range a-particles, improves OS, and is a good treatment option for patients with CRPC and symptomatic bone metastases.28,29,30 Denosumab, a human anti-RANKL monoclonal antibody, can delay bone metastasis in men with PCa.31,32 According to recent guidelines, the first-line treatments for MCRPC include abiraterone acetate plus prednisone (AA/P), enzalutamide, radium 223, docetaxel, and sipuleucel-T. Cabazitaxel, AA/P, enzalutamide, and radium have been approved as second-line treatments for CRPC following docetaxel treatment.6,7,13,28,33,34,35
Although men with MCRPC benefit from all of these new therapies, a significant proportion of patients exhibit primary resistance to these agents, and virtually all patients develop secondary resistance.36,37 The resistance mechanisms of tumors to AR blocking agents, including AR protein overexpression, AR gene amplification, AR gene mutations, and AR variants (AR-Vs), are directly related to the activation of AR-dependent pathways38,39 and processes independent of the AR signaling pathway. The reported mechanisms of resistance to taxanes are related to several contributing factors, such as tubulin alterations, multidrug-resistance (MDR) kinesin overexpression, signaling pathways and cytokines related to epithelial-mesenchymal transition (EMT), ETS fusion gene family members, and AR splice variants (AR-SVs).[40] Putative predictive biomarkers, including AR-SVs, homologous recombination (HR) repair defects including BRCA2 loss, mismatch repair (MMR) defects, and phosphatase and tensin homolog (PTEN) loss, would also benefit the treatment of the disease.[41] Comprehensive and integrative genomic analysis of MCRPC could make individualized targeted therapies possible and help to develop new drugs.[42] Olaparib was the first poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) inhibitor to be tested in men with PCa and could prolong OS in patients with PARP mutations.43,44,45 Other PARP inhibitors (rucaparib and niraparib) are still being tested in clinical trials.46,47 Pembrolizumab, an anti-PD 1 antibody, might be useful in advanced PD-L1-positive PCa.48,49,50,51 The results of clinical trials treating MCRPC with another anti-PD 1 antibody, atezolizumab, were reported recently.52,53,54,55 Radioligand therapy (RLT) with [Lu-177] Lu-PSMA-617 (Lu-PSMA) is a novel targeted therapy that seems to be safe, and this therapy prolong overall survival in men with MCRPC.56,57,58,59,60 Ipatasertib (an AKT inhibitor) in combination with abiraterone acetate might be active against tumors with PIK3CA mutations or PTEN loss in men with MCRPC.[61] There are other therapies (veliparib and ipilimumab) for MCRPC in clinical trials not mentioned above.62,63,64,65Thus, sequencing-based tumor analyses could identify specific genomic aberrations and guide individualized therapy.[41] To implement precision cancer medicine effectively, more predictive or prognostic biomarkers (such as circulating tumor cells [CTC][66] or AR-V7[67]) are being developed to predict the therapeutic efficiency or resistance to therapy as well as patient prognosis.68,69
Bibliometric analysis is an effective method for analyzing scientific publications and identifying the trends of present or future studies. This approach has been widely used in the area of medicine.70,71,72 MCRPC remains incurable and is a hot topic in the field of urology. An increasing number of articles and reviews related to the therapies, cellular mechanisms, and other aspects of MCRPC have been published, but a quantitative description of the publications on MCRPC is lacking. A comprehensive bibliometric study was conducted on this subject. This study aimed to provide an overview of MCRPC from 1979 to 2018, explore clusters and trends in research, and investigate the future direction of MCRPC research.
2. Method and data
This bibliometric study analyzed the papers related to “metastatic castration-resistant prostate cancer” obtained from the Science Citation Index-Expanded (SCI-E) that were published during the period from 1979 to 2018 (the year 1979 was chosen as a starting point when the first paper was published[73]). The data were acquired through the Web of Science (WoS) Core Collection database by searching the “topic” field, which included the title, abstract, and keyword fields, on 4 November 2018. The search strategy for the WoS database was (TS =(“castrat∗ resistant” or “hormone refractory” or “hormone independent” or “hormone resistant” or “hormone insensitive” or “androgen independent” or “androgen insensitive” or “androgen refractory” or “castration recurrent” or “androgen resistant” or “castration refractory”) and metastatic and (“prostat∗ Neoplasm∗” or “prostat∗ Tumor∗” or “prostat∗ Cancer∗” or “prostat∗ carcinoma∗” or “prostat∗ adenocarcinoma∗”) or “MCRPC” or “metastatic CRPC” or “metastatic neuroendocrine prostate cancer” OR “metastatic NEPC”). The papers originating from England, Scotland, Northern Ireland, and Wales were classified as belonging to the United Kingdom (UK), while those from Macao, Taiwan, and Hong Kong were not grouped under the China heading.
A total of 6304 publications, including 118 SCI-E highly cited articles and 4 SCI-E hot articles, matched the choice criteria listed above across 13 document types. The 13 document types were article (3304), meeting abstract (1883), review (785), editorial material (201), proceedings paper (123), letter (82), correction (29), news item (10), note (9), data paper (5), book chapter (4), retracted publication (3), and retraction (1). Of the 6304 publications, a total of 6124 (97.145%) were published in English. The other eight languages were German (83; 1.317%), French (65; 1.031%), Spanish (25; 0.397%), Hungarian (3; 0.048%), Korean (1; 0.016%), Polish (1; 0.016%), Welsh (1; 0.016%), and Turkish (1; 0.04%). Thus, English was the predominant language of the academic publications on MCRPC research. Only articles and reviews were further analyzed because they not only comprised the majority of the publications but also were peer-reviewed. Meeting abstracts were not evaluated because they tend to reflect organizational logistics rather than editorial decisions.[74] The impact factors (IFs) of the WoS journals were determined from the 2017 Journal Citation Reports (JCR). Derwent Data Analyzer (DDA) software was used to assess the data and analyze journals, keywords, and international cooperation.
3. Results and discussion
3.1. The performance of related publications and countries
Seventy-six countries contributed 6304 publications to the MCRPC research field between 1979 and 2018, indicating that MCRPC is a global health problem that attracts worldwide attention. During the first year, there was only one published study within the WoS, and annual publication numbers grew slowly in the first 20 years (Fig. 1). During the next 10 years, the number of annual publications increased from 66 (2000) to 187 (2009). In the last 9 years (2010–2018), the annual publication number has increased rapidly, rising from 189 (2010) to 606 (2018). Thus, MCRPC has attracted increasing attention and has become one of the hottest research fields in cancer research.
Figure 1.
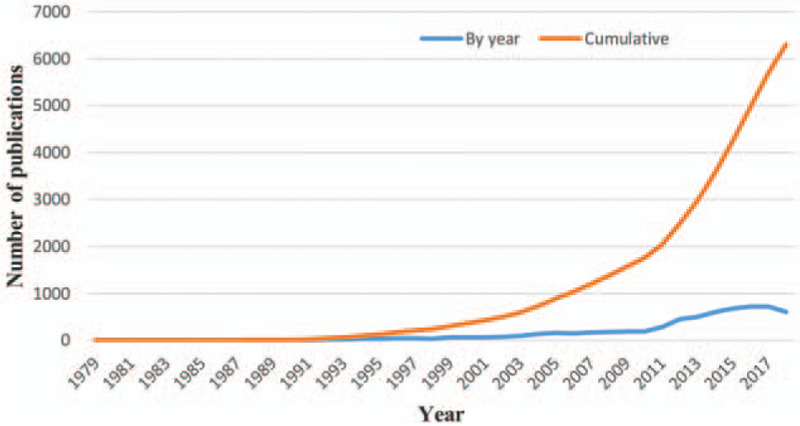
Trends in the number of publications related to MCRPC by year.
3.2. Cooperation of countries/territories
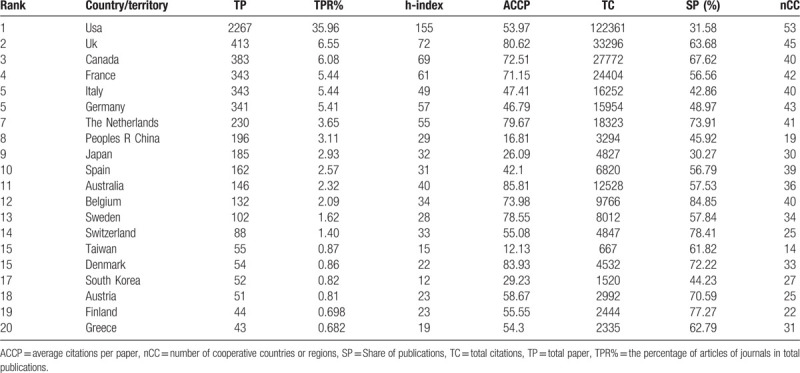
Publications on MCRPC between 1979 and 2018 came from 76 countries. The top 20 most productive countries/territories in the MCRPC research field are shown in Table 1. The USA headed the list, with a publication share of 35.96% and the highest h-index (155). The UK ranked second, followed by Canada, France, Italy and Germany. The Netherlands, mainland China, Japan, and Spain were ranked in positions 6–10. The remaining 20 most productive countries/territories were mostly in Europe. Economic development seemed to not only increase the incidence of PCa75,76 but also contribute to high scientific and academic investments, as nearly all of the 20 most productive countries/territories were economically prosperous. In Western countries, the incidence of PCa was higher than that in developing countries. The first reason for this difference was that the men in Western countries had more access to medical care, including screening and early detection.[77] The second reason was that the incidence of PCa was related to environmental, dietary, and genetic factors.78,79,80
Table 1.
Contribution and impact of the top 20 most productive countries/territories in MCRPC research.
DDA software was used to draw a network diagram from a cooccurrence matrix.[81] Each node represented a different country, and node size corresponded to the number of publications. Similarly, the lines connecting the countries represented their cooperation, and the line thickness indicated the frequencies of that collaboration. The academic collaboration network of the top 20 most productive countries/territories is shown in Figure 2. The USA was the center of this collaboration network and the leader of MCRPC research in cooperation with the other 53 countries/territories. Asian countries/territories such as mainland China, Japan, South Korea, and Taiwan had smaller collaboration networks than European countries and North American countries, might explain why Asian countries/territories had smaller h-indexes, TCs, and ACCPs.[82] This network might also suggest that collaborations benefit the number, impact, and quality of the papers.83,84
Figure 2.
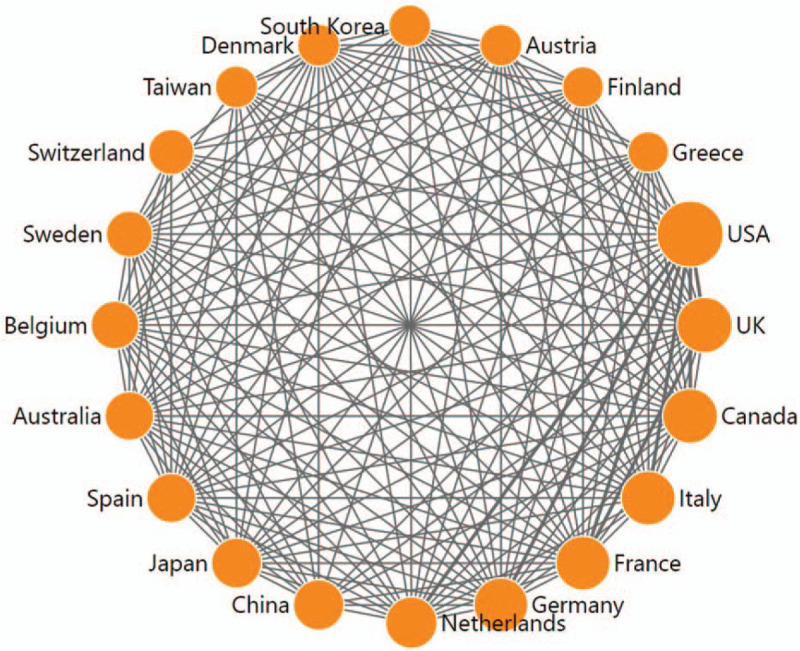
Collaborative relationships among the top 20 most productive countries/territories.
3.3. Institute contributions to publications
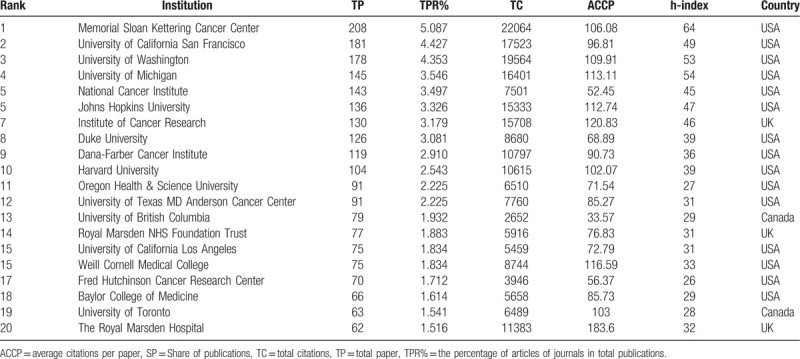
The top 20 most productive institutes from 1979 to 2018 are listed in Table 2: three of these institutes are based in the UK, two are in Canada, and the rest are in the USA. This distribution reiterated the predominance of the USA in the research field of MCRPC. As the most historical and largest private cancer center in the world, Memorial Sloan Kettering Cancer Center contributed 208 publications, more than any other institution, accounting for 5.087% of the world's publications in this field, and these publications were accompanied by 22,064 citations. The University of California San Francisco was second in productivity, with 181 publications and a total of 17,523 citations. The University of Washington and the University of Michigan ranked third and fourth, respectively. Regarding the h-index, Memorial Sloan Kettering Cancer Center also ranked first, followed by the University of Michigan and University of Washington. The institutions with high h-indexes were mainly from the USA, the UK and Canada, indicating that these countries had outstanding academic institutions and capabilities in this field of MCRPC.
Table 2.
The top 20 most productive institutions of publication, citations, and h-index.
3.4. Contributions of leading authors
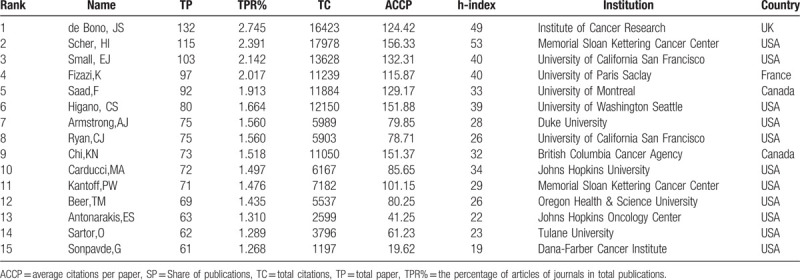
Many scientists from a wide range of origins have researched MCRPC and published their findings in the WoS. The top 15 authors in MCRPC research are listed in Table 3. The most prolific author on the topic was de Bono, JS, who published 132 scientific articles and had a total of 16,423 citations. In addition, the author with the highest number of citations and the most citations per article published was Scher, HI, who had 115 articles and 17,978 citations. In the top 15 authors, de Bono, JS was the only author from the UK; Fizazi, K was from France; Saad, F and Chi, KN were from Canada; Scher, HI and the other top 10 scientists were American. These institutions where these scientists worked were generally among the top 20 most productive institutions. For example, de Bono, JS worked at the Institute of Cancer Research, and Scher, HI worked at Memorial Sloan Kettering Cancer Center. In addition to these top scientists, there were more than 16,000 scientists in the world researching MCRPC.
Table 3.
Contribution of the top 15 authors in MCRPC research.
3.5. Contributions in leading research areas and journals
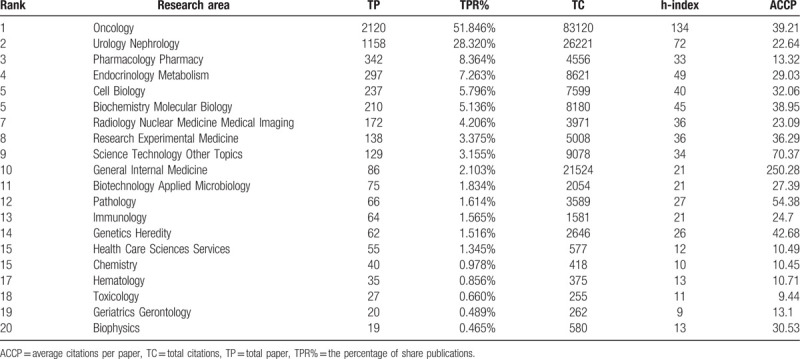
A total of 4089 articles were published in the MCRPC research field, and these articles involved 52 research areas. The distribution of the top 20 research areas in MCRPC research is listed in Table 4. “Oncology” was undoubtedly the dominant research area, with 2120 articles, followed by “urology nephrology,” “pharmacology pharmacy,” “endocrinology metabolism,” “cell biology,” and “biochemistry molecular biology.” There were 2120 publications in the area of “oncology,” comprising 51.85% of the total publications, and “oncology” had the highest h-index (134). This analysis illustrated that research hotspots were correlated with the prevention, diagnosis, and treatment of cancer. “Pharmacology pharmacy” ranked third, indicating that therapy was an essential area of MCRPC research. All indicated the importance of treatments such as chemotherapy, new hormone therapy, immunotherapy, radiotherapy, and targeted therapy in the MCRPC research field.
Table 4.
Contribution of the top 20 research areas in MCRPC research.
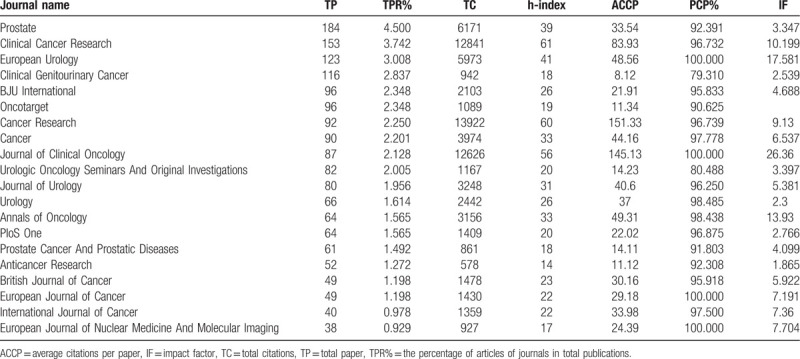
A total of 4089 articles were published in 595 journals, with 257 journals publishing only one article and 111 journals publishing only two articles. There are 20 journals identified in Table 5, and their MCRPC publication numbers, total citations, average citations per item, h-indexes, and impact factors (impact factors were obtained from JCR 2017) are also listed. The journal Prostate headed the list with a total number of 184 publications, followed by the journal Clinical Cancer Research (153), the journal European Urology (123), and the journal Clinical Genitourinary Cancer (116). The sixth place journal Oncotarget was excluded from the WoS in 2017; thus, its IF was unknown.
Table 5.
Contribution of the top 20 most productive Journals in MCRPC research.
A bubble chart was also used to show the trend in the publications.[85] The trend of the top 20 productive journals by year is displayed in Figure 3. There were quite a few articles published in the top 20 productive journals from 1981 to 2000. During 2001–2010, the publications in these journals increased steadily, similar to the pattern for total publication numbers shown in Figure 1. Since 2010, the annual number of articles in most journals, such as Prostate, Clinical Cancer Research, European Urology, and Clinical Genitourinary Cancer, has increased rapidly, as shown in Figure 3. Annual papers of partial journals remained relatively steady, such as the journal Cancer Research, the journal Cancer, and the Journal of Clinical Oncology.
Figure 3.
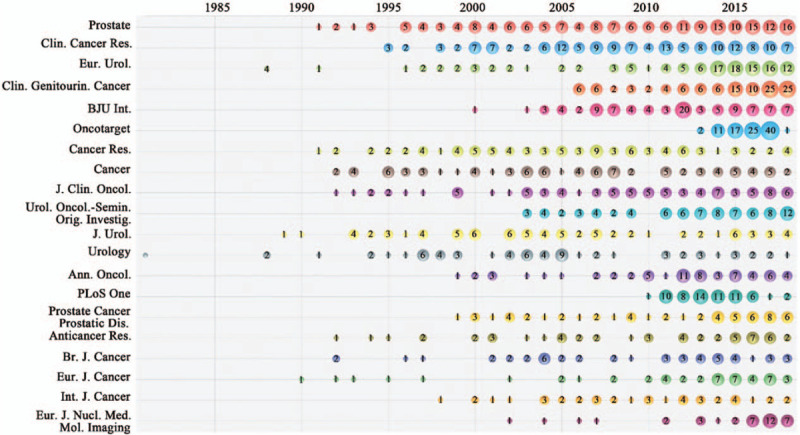
Bubble chart of the top 20 productive journals by year.
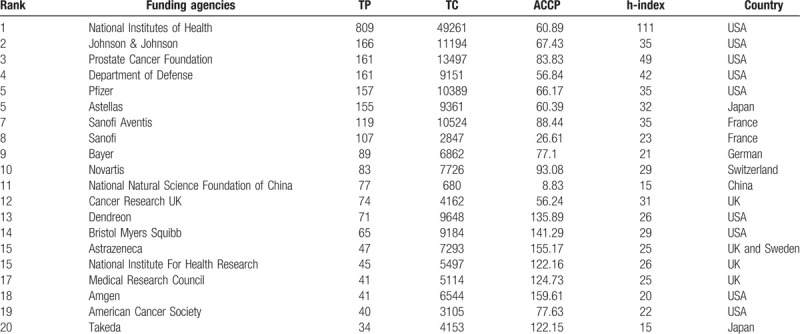
3.6. Contributions of funding agencies
Scientific productivity is related to research and development expenditures.86,87,88 The distribution of the top 20 most productive funding agencies for MCRPC research is displayed in Table 6. The National Institutes of Health (NIH) topped the list with 809 funded articles and the highest h-index (111). The pharmaceutical company Johnson & Johnson ranked second with 166 funded articles. The Prostate Cancer Foundation (PCF) ranked third with 161 funded articles. Pfizer (157) and Astellas (155) followed the Department of Defense (DOD), which funded 161 articles. The National Natural Science Foundation of China (NNSFC) ranked 11th with 77 funded articles and an h-index of 15. Twelve of these 20 funding agencies were global pharmaceutical companies, and the remaining 8 were state-funded institutions or charitable foundations. The NIH was the world's largest funder of biomedical research and invested approximately $30 billion per year in biomedical research.[89] The PCF was the world's leading philanthropic organization funding and accelerating PCa research, and it raised more than $765 million and funded more than 2000 research programs at nearly 210 cancer centers and universities.[90] The NNSFC, the largest natural science foundation in China, invested nearly $1.1 billion in biomedical research in 2018.[91] Compared with the NIH and PCF, the NNSFC needs to expend more efforts to not only increase research sponsorship funds but also improve the quality of academic outputs.
Table 6.
Contribution of the top 20 most productive funding agencies in MCRPC research.
The influence of commercial interests on medical science was worldwide and essential.[92] The discovery of new medications, devices, and techniques was always funded primarily by for-profit companies.[93] For example, abiraterone acetate was first reported in the 1990s,[94] and until 2009, the rights for the commercialization of abiraterone acetate were held by Johnson & Johnson, which conducted ongoing clinical trials to expand the clinical uses of abiraterone acetate. With the efforts of Johnson & Johnson, abiraterone acetate became a standard treatment for CRPC and MCRPC. The commercialization of other drugs followed similar patterns. However, biomedical research funded by for-profit companies can be influenced in important ways.[95] The sponsorship of drug or device studies by the manufacturing company led to more favorable efficacy results and conclusions than sponsorship by other sources.96,97,98 Even the professional medical associations (PMAs) playing an essential role in defining and advancing health care standards could be influenced by pharmaceutical and device companies.[99] Sometimes irregularities at those companies, such as refusing to provide all the research data to the study team,[100] reporting partial data as the primary outcome, incomplete reporting of some adverse events,101,102,103 or concealing some clinical trial data showing harm,[104] were reported. In addition, the worst breach of trust was that clinical trial manuscripts authored by sponsor employees attributed first authorship to investigators who did not always disclose their industry-based financial support.[105] This issue was also the reason that authors were required to detail their competing interests.
3.6.1. Analysis of keywords
Keyword analysis offers information on how authors conceptualize their own research, and keywords have been vital for monitoring the development of science.85,106,107,108 However, our keyword analysis also had a limitation: papers without author keywords were excluded from the analysis. Keywords appearing in the MCRPC articles from 1979 to 2018 were analyzed and ranked. The top 30 author keywords by year are displayed in Figure 4. Apart from the most frequently used searching keywords “prostate cancer” and “castration-resistant prostate cancer,” the other keywords that frequently appeared at the same time were “docetaxel” (330, ranking 3rd), “abiraterone” (321, ranking 4th), “castration-resistant” (321, ranking 5th), “metastatic castration-resistant prostate cancer” (275, ranking 6th), “metastasis” (274, ranking 7th), “chemotherapy” (263, ranking 8th), “androgen receptor” (ranking 9th) and “enzalutamide” (ranking 10th). Obviously, some author keywords were related to MCRPC therapy, such as “docetaxel,” “abiraterone,” and “cabazitamide.” Docetaxel and cabazitaxel are taxane chemotherapy agents. Abiraterone and enzalutamide are ADT agents. The articles related to “docetaxel,” ”abiraterone,” “chemotherapy,” “enzalutamide,” “immunotherapy,” “cabazitaxel,” or “radium-223” sharply increased after 2010. This increase illustrated that the new therapies became topics of interest once they were approved by the US Food and Drug Administration (FDA); however, the increase in docetaxel publications did not match this trend since docetaxel was approved by the FDA in 2004.[109]
Figure 4.
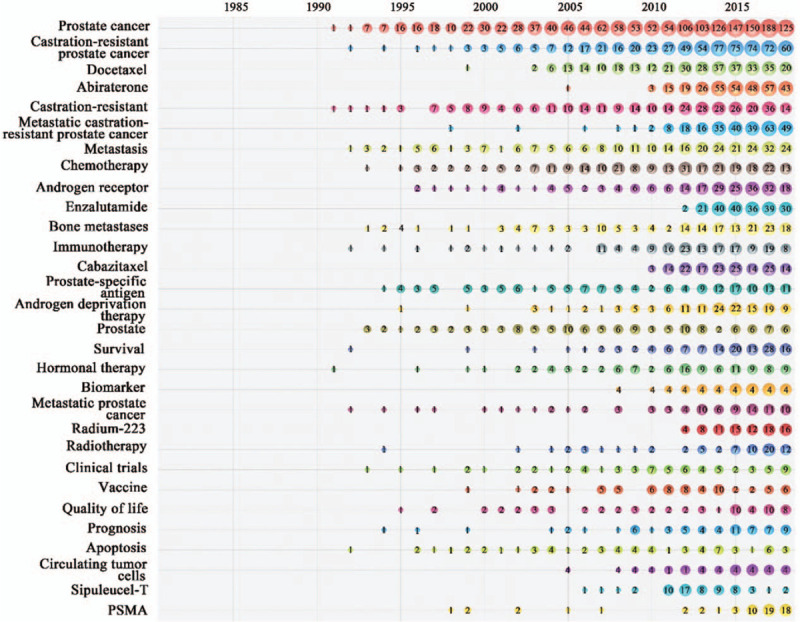
Bubble chart of top 30 author keywords by year.
3.6.2. Research trends and future hotspots
Given the results in Figure 4, new therapies appear to become areas of interest once they are reported. Therapies that target critical cellular mechanisms of drug resistance are considered to be the most promising approaches for MCRPC therapy.[11] Many new drugs (such as darolutamide[110] and apalutamide[111]) targeting androgen signaling are being tested in clinical trials and will hopefully be effective in abiraterone- or enzalutamide-resistant patients. Emergence Indicators in DDA8 software have been proved useful to spotlight “hot” research topics within the domain.112,113 The scores are calculated from four criteria—Novelty, Persistence, Growth and Community[114]—and represent the accuracy of predicting the research trends in the next few years. The emergence scores of 15 author keywords are displayed in Table 7. “Prostate-specific membrane antigen (PSMA)” achieved the second highest emergence score of 10.504, followed by “Radiotherapy” (5.862), “Radium-223” (5.04), “Liquid biopsy” (4.907) and “Bone metastases” (4.613).
Table 7.
The emergence scores in MCRPC field.
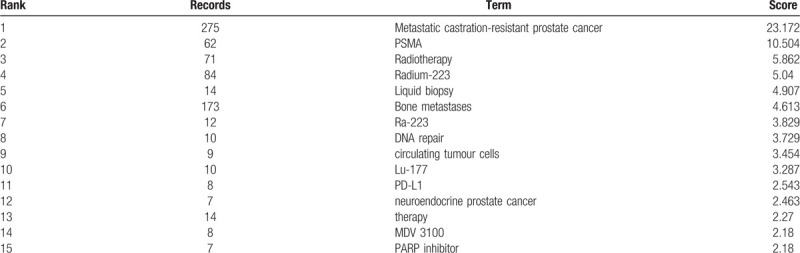
PSMA is frequently overexpressed and strongly upregulated in PCa, making PSMA-based RLT a promising treatment for MCRPC.115,116,117,118 RLT with [Lu-177] Lu-PSMA-617 (Lu-PSMA) was first reported in 2015[57] and attracted the most scholarly attention due to its low toxicity profile and respectable response rates.57,59,119,120 Liquid biopsy is widely applied to cancer research and will play a vital role in predicting the therapeutic efficiency or resistance to therapy.121,122,123 “DNA repair” ranked 8th with an emergence score of 3.729. DNA repair defects might present another promising treatment opportunity for patients with MCRPC,[124] treatments that might include PARP inhibition[43] and platinum chemotherapy.[125] Research on bone metastasis has also attracted considerable attention, and radium 223 is the most promising treatment for patients with bone metastases. AR and the AR signaling pathway remain the principal drivers of the development and progression of MCRPC, making AR and its signaling pathway the main targets of principal therapeutic approaches.[126] The drug resistance or cross-resistance of therapies might affect drug sequence choices in MCRPC,[127] making drug sequences a promising hotspot in the future.
3.7. An Analysis of the most cited papers
Although multiple indicators were used to evaluate the impact of scientific publications, citation counts are still an important measurement of influence in this research field.120,121 The top 20 most cited publications in the MCRPC research field during 1981–2018 are presented in Table 8. The most highly cited paper was “Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer.” This article was published in the New England Journal of Medicine in 2010 by Kantoff, PW and headed the lists of total citations (2715) and annual citations (339.36). “Abiraterone and Increased Survival in Metastatic Prostate Cancer,” authored by de Bono, JS et al, took second place, with 2045 total citations and 292.14 annual citations. “Increased Survival with Enzalutamide in Prostate Cancer after Chemotherapy,” authored by Scher, HI et al, ranked third with 1857 total citations and 309.5 annual citations.
Table 8.
The top 20 most cited publications in MCRPC research field during 1979-2018.
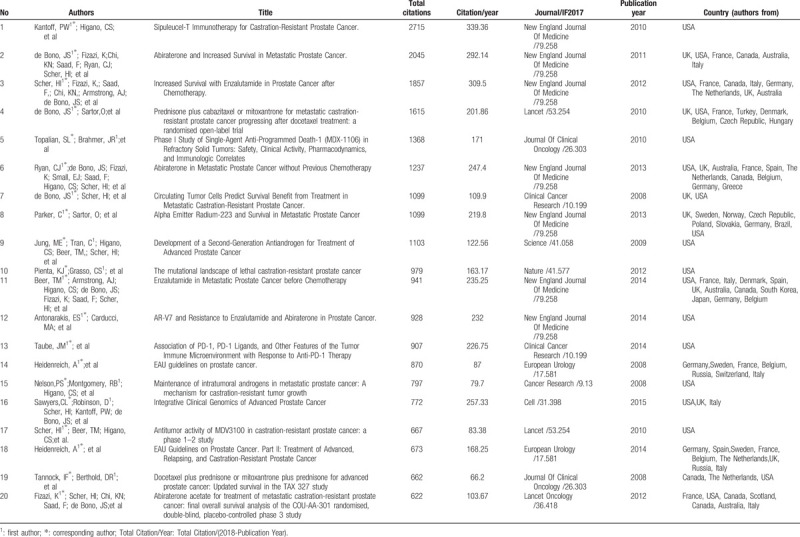
Among these top 20 papers, 7 were published in the New England Journal of Medicine; 2 were published in Lancet; 2 were published in the Journal of Clinical Oncology; 2 were published in European Urology;2 were published in Clinical Cancer Research; and Nature, Science, Cell, Lancet Oncology and Cancer Research each published 1 of the top articles. Eight papers had only American authors, and the remaining 12 papers had authors from more than 2 countries, meaning they were papers that resulted from international cooperation. Fifteen of the top scientists, such as de Bono, JS and Scher, HI, participated in 14 papers in a cooperative manner. This finding illustrated that the top scientists played a key role in the development of the entire field and that collaborations benefited the number, impact and quality of papers. Most of the top 20 papers were related to therapies for MCRPC, demonstrating that therapy was the most critical area of MCRPC research.
4. Conclusions
In this paper, a bibliometric analysis was performed to evaluate the trends in MCRPC research during 1979–2018. The results showed that the publications in MCRPC research increased significantly after 2010, possibly due to new therapies, such as abiraterone and enzalutamide. The findings illustrated that the USA dominated the MCRPC research in the areas of total publications, top institutions, top scientists and most cited papers. Some information could be obtained from this study:
-
(1)
MCRPC has attracted increasing attention and has become a worldwide health issue.
-
(2)
Compared with the USA, Asian countries, especially mainland China, are required to exert more effort in the areas of research funding and international collaboration to improve the impact and quality of their publications.
-
(3)
When facing drug resistance, combined therapies might improve quality of life and extend survival. Understanding the cellular mechanisms would help the development of new drugs that overcome existing resistance. Liquid biopsy will play a vital role in predicting the therapeutic efficiency or resistance to therapy.
-
(4)
Therapies that target critical cellular mechanisms of drug resistance, especially PSMA-based RLT and new therapies targeting DNA repair or the AR signing pathway, could be the next hotpot.
-
(5)
Research on bone metastasis has also attracted considerable attention, and radium 223 is the most promising treatment for patients with bone metastases.
This study will help researchers understand the global overview of MCRPC, find potential collaborators in Western countries and grasp the promising attractive areas of MCRPC research.
Author contributions
Conceptualization: Yuehua Wan, Huadong He.
Data curation: Hui Fang.
Formal analysis: Hui Fang, Yuehua Wan, Yanqi Wu.
Funding acquisition: Chao Chen.
Investigation: Lugeng He, Chao Chen, Yuyong Wang, Hongwei Ge, Lili Wang.
Methodology: Huadong He.
Project administration: Yuehua Wan, Huadong He.
Resources: Lili Wang.
Visualization: Hongwei Ge.
Writing – original draft: Lugeng He.
Writing – review & editing: Yuehua Wan.
Lugeng He: 0000-0001-5059-9005.
Footnotes
How to cite this article: He L, Fang H, Chen C, Wu Y, Wang Y, Ge H, Wang L, Wan Y, He H. Metastatic castration-resistant prostate cancer: Academic insights and perspectives through bibliometric analysis. Medicine. 2020;99:15(e19760).
Abbreviations: AA/P = abiraterone acetate plus prednisone, ACCP = average citations per paper, AR = androgen receptor, CTC = circulating tumor cells, DDA = Derwent Data Analyzer, FDA = Food and Drug Administration, MCRPC = metastatic castration-resistant prostate cancer, nCC = number of cooperative countries or regions, OS = overall survival, PSMA = prostate-specific membrane antigen, RLT = radioligand therapy, SP = Share of publications, TC = total citations, TP = total paper, TPR% = the percentage of articles of journals in total publications, WoS = Web of Science.
This work is financially supported by the Natural Science Foundation of Zhejiang Province (No. LY18H160063) and the Zhejiang Medical Science and Technology Project (No. 2019RC241).
The authors have no conflicts of interest to disclose.
References
- [1]. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. Ca-a Cancer Journal for Clinicians 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2]. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115–32. [DOI] [PubMed] [Google Scholar]
- [3]. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–403. [DOI] [PubMed] [Google Scholar]
- [4]. Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol 2008;53:68–80. [DOI] [PubMed] [Google Scholar]
- [5]. Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol 2017;71:618–29. [DOI] [PubMed] [Google Scholar]
- [6]. Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU Guidelines on Prostate Cancer. Part II: Treatment of Advanced, Relapsing, and Castration-Resistant Prostate Cancer. Eur Urol 2014;65:467–79. [DOI] [PubMed] [Google Scholar]
- [7]. Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol 2017;71:630–42. [DOI] [PubMed] [Google Scholar]
- [8]. Ahmad K. New progress in treatment of hormone-refractory prostate cancer. Lancet Oncol 2004;5:706. [DOI] [PubMed] [Google Scholar]
- [9]. Petrylak DP. Future directions in the treatment of androgen-independent prostate cancer. Urology 2005;65:8–12. [DOI] [PubMed] [Google Scholar]
- [10]. Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res 2008;68:4447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Seruga B, Ocana A, Tannock IF. Drug resistance in metastatic castration-resistant prostate cancer. Nat Rev Clin Oncol 2011;8:12–23. [DOI] [PubMed] [Google Scholar]
- [12]. Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract 2011;65:1180–92. [DOI] [PubMed] [Google Scholar]
- [13]. Basch E, Loblaw DA, Oliver TK, et al. Systemic Therapy in Men With Metastatic Castration-Resistant Prostate Cancer: American Society of Clinical Oncology and Cancer Care Ontario Clinical Practice Guideline. J Clin Oncol 2014;32:3436–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 2010;376:1147–54. [DOI] [PubMed] [Google Scholar]
- [15]. Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol 2008;26:242–5. [DOI] [PubMed] [Google Scholar]
- [16]. Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet 2010;375:1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–97. [DOI] [PubMed] [Google Scholar]
- [18]. Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur Urol 2017;71:151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Tran C, Ouk S, Clegg NJ, et al. Development of a Second-Generation Antiandrogen for Treatment of Advanced Prostate Cancer. Science 2009;324:787–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N Engl J Med 2014;371:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Ryan CJ. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy (vol 368, pg 138, 2013). N Engl J Med 2013;368:584–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. de Bono JS, Bianchini D, Zivi A. Abiraterone and Increased Survival in Metastatic Prostate Cancer REPLY. N Engl J Med 2011;365:767–8. [DOI] [PubMed] [Google Scholar]
- [23]. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152–60. [DOI] [PubMed] [Google Scholar]
- [24]. Kawalec P, Paszulewicz A, Holko P, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. A systematic review and meta-analysis. Arch Med Sci 2012;8:767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in Prostate Cancer: The First FDA-Approved Therapeutic Cancer Vaccine. Clin Cancer Res 2011;17:3520–6. [DOI] [PubMed] [Google Scholar]
- [26]. Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol 2006;24:3089–94. [DOI] [PubMed] [Google Scholar]
- [27]. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med 2010;363:411–22. [DOI] [PubMed] [Google Scholar]
- [28]. Parker C, Nilsson S, Heinrich D, et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N Engl J Med 2013;369:213–23. [DOI] [PubMed] [Google Scholar]
- [29]. Sartor O, Coleman R, Nilsson S, et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 2014;15:738–46. [DOI] [PubMed] [Google Scholar]
- [30]. Saad F, Carles J, Gillessen S, et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol 2016;17:1306–16. [DOI] [PubMed] [Google Scholar]
- [31]. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 2012;379:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol 2015;26:368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Noonan KL, North S, Bitting RL, et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol 2013;24:1802–7. [DOI] [PubMed] [Google Scholar]
- [34]. Fizazi K, Scher HI, Molina A, et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2012;13:983–92. [DOI] [PubMed] [Google Scholar]
- [35]. Sonpavde G, Bhor M, Hennessy D, et al. Sequencing of Cabazitaxel and Abiraterone Acetate After Docetaxel in Metastatic Castration-Resistant Prostate Cancer: Treatment Patterns and Clinical Outcomes in Multicenter Community-Based US Oncology Practices. Clin Genitourin Cancer 2015;13:309–18. [DOI] [PubMed] [Google Scholar]
- [36]. Karantanos T, Corn PG, Thompson TC. Prostate cancer progression after androgen deprivation therapy: mechanisms of castrate resistance and novel therapeutic approaches. Oncogene 2013;32:5501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Boudadi K, Antonarakis ES. Resistance to Novel Antiandrogen Therapies in Metastatic Castration-Resistant Prostate Cancer. Clin Med Insights Oncol 2016;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Luo J, Attard G, Balk SP, et al. Role of Androgen Receptor Variants in Prostate Cancer: Report from the 2017 Mission Androgen Receptor Variants Meeting. Eur Urol 2018;73:715–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012;487:239–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Giacinti S, Poti G, Roberto M, et al. Molecular Basis of Drug Resistance and Insights for New Treatment Approaches in mCRPC. Anticancer Res 2018;38:6029–39. [DOI] [PubMed] [Google Scholar]
- [41]. Sumanasuriya S, De Bono J. Treatment of Advanced Prostate Cancer-A Review of Current Therapies and Future Promise. Cold Spring Harb Perspect Med 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161:1215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med 2015;373:1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Reichert Z, Carneiro BA, Daignault-Newton S, et al. A randomized phase II trial of abiraterone, olaparib or abiraterone plus olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair defects. J Clin Oncol 2017;35. [Google Scholar]
- [45]. Goodall J, Mateo J, Yuan W, et al. Circulating Cell-Free DNA to Guide Prostate Cancer Treatment with PARP Inhibition. Cancer Discov 2017;7:1006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Rescigno P, Chandler R, de Bono J. Relevance of poly (ADP-ribose) polymerase inhibitors in prostate cancer. Curr Opin Support Palliat Care 2018;12:339–43. [DOI] [PubMed] [Google Scholar]
- [47]. Baciarello G, Gizzi M, Fizazi K. Advancing therapies in metastatic castration-resistant prostate cancer. Expert Opin Pharmacother 2018;19:1797–804. [DOI] [PubMed] [Google Scholar]
- [48]. Hansen AR, Massard C, Ott PA, et al. Pembrolizumab for advanced prostate adenocarcinoma: findings of the KEYNOTE-028 study. Ann Oncol 2018;29:1807–13. [DOI] [PubMed] [Google Scholar]
- [49]. McNeel DG, Eickhoff JC, Jeraj R, et al. DNA vaccine with pembrolizumab to elicit antitumor responses in patients with metastatic, castration-resistant prostate cancer (mCRPC). J Clin Oncol 2017;35. [Google Scholar]
- [50]. Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 Ligands, and Other Features of the Tumor Immune Microenvironment with Response to Anti-PD-1 Therapy. Clin Cancer Res 2014;20:5064–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52]. Vaishampayan UN. Changing face of metastatic prostate cancer: the law of diminishing returns holds true. Curr Opin Oncol 2017;29:196–200. [DOI] [PubMed] [Google Scholar]
- [53]. Powles T, Fizazi K, Gillessen S, et al. A phase III trial comparing atezolizumab with enzalutamide vs enzalutamide alone in patients with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2017;35. [Google Scholar]
- [54]. Fong L, Morris M, Armstrong A, et al. A phase Ib trial to study the safety and tolerability of atezolizumab with radium-223 dichloride in patients with metastatic castrate-resistant prostate cancer (mCRPC). Cancer Res 2017;77. [Google Scholar]
- [55]. Kim JW, Shaffer DR, Massard C, et al. A phase Ia study of safety and clinical activity of atezolizumab (atezo) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2018;36. [Google Scholar]
- [56]. Ferdinandus J, Violet J, Sandhu S, et al. Prostate-specific membrane antigen theranostics: therapy with lutetium-177. Curr Opin Urol 2018;28:197–204. [DOI] [PubMed] [Google Scholar]
- [57]. Ahmadzadehfar H, Rahbar K, Kurpig S, et al. Early side effects and first results of radioligand therapy with Lu-177-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res 2015;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58]. Ahmadzadehfar H, Eppard E, Kurpig S, et al. Therapeutic response and side effects of repeated radioligand therapy with Lu-177-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget 2016;7:12477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Rahbar K, Bogeman M, Yordanova A, et al. Delayed response after repeated Lu-177-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur J Nucl Med Mol Imaging 2018;45:243–6. [DOI] [PubMed] [Google Scholar]
- [60]. Ahmadzadehfar H, Wegen S, Yordanova A, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using Lu-177 Lu-PSMA-617. Eur J Nucl Med Mol Imaging 2017;44:1448–54. [DOI] [PubMed] [Google Scholar]
- [61]. de Bono J, Bracarda S, Chi K, et al. Randomized phase III trial of ipatasertib vs. placebo, plus abiraterone and prednisone/prednisolone, in men with asymptomatic or mildly symptomatic previously untreated metastatic castrate-resistant prostate cancer (mCRPC). Ann Oncol 2017;28:v269–94. [Google Scholar]
- [62]. Hussain M, Carducci MA, Slovin S, et al. Targeting DNA repair with combination veliparib (ABT-888) and temozolomide in patients with metastatic castration-resistant prostate cancer. Invest New Drugs 2014;32:904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Hussain M, Daignault S, Twardowski P, et al. Abiraterone plus prednisone (Abi) plus /- veliparib (Vel) for patients (pts) with metastatic castration resistant prostate cancer (CRPC): NCI 9012 updated clinical and genomics data. J Clin Oncol 2017;35:5001. [Google Scholar]
- [64]. Pahuja S, Appleman LJ, Belani CP, et al. Preliminary activity of veliparib (V) in BRCA2-mutated metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2015;33:170. [Google Scholar]
- [65]. Beer TM, Kwon ED, Drake CG, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol 2017;35:40–7. [DOI] [PubMed] [Google Scholar]
- [66]. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302–9. [DOI] [PubMed] [Google Scholar]
- [67]. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014;371:1028–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Armstrong AJ, Saad F, Phung D, et al. Clinical Outcomes and Survival Surrogacy Studies of Prostate-Specific Antigen Declines Following Enzalutamide in Men with Metastatic Castration-Resistant Prostate Cancer Previously Treated with Docetaxel. Cancer 2017;123:2303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Chi KN, Kheoh T, Ryan CJ, et al. A prognostic index model for predicting overall survival in patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate after docetaxel. Ann Oncol 2016;27:454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70]. Begum M, Lewison G, Lawler M, et al. Mapping the European cancer research landscape: An evidence base for national and Pan-European research and funding. Eur J Cancer 2018;100:75–84. [DOI] [PubMed] [Google Scholar]
- [71]. Tran BX, McIntyre RS, Latkin CA, et al. The Current Research Landscape on the Artificial Intelligence Application in the Management of Depressive Disorders: A Bibliometric Analysis. Int J Environ Res Public Health 2019;16:2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72]. Tran BX, Ho RCM, Ho CSH, et al. Depression among Patients with HIV/AIDS: Research Development and Effective Interventions (GAP(RESEARCH)). Int J Env Res Pub He 2019;16:1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73]. Cox EB, Berry WR. Ldh as a Predictor of Chemotherapy Response and Survival in Hormone Refractory Metastatic Prostate-Cancer (Hrpc). P Am Assoc Canc Res 1979;20:414–514. [Google Scholar]
- [74]. Popkirov S, Jungilligens J, Schlegel U, et al. Research on dissociative seizures: A bibliometric analysis and visualization of the scientific landscape. Epilepsy Behav 2018;83:162–7. [DOI] [PubMed] [Google Scholar]
- [75]. Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016 Featured Updates to the NCCN Guidelines. J Natl Compr Cancer Netw 2016;14:19–30. [Google Scholar]
- [76]. Klein J, von dem Knesebeck O. Socioeconomic inequalities in prostate cancer survival: A review of the evidence and explanatory factors. Soc Sci Med 2015;142:9–18. [DOI] [PubMed] [Google Scholar]
- [77]. Rebbeck TR. Prostate Cancer Genetics: Variation by Race, Ethnicity, and Geography. Semin Radiat Oncol 2017;27:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78]. Cuzick J, Thorat MA, Andriole G, et al. Prevention and early detection of prostate cancer. Lancet Oncol 2014;15:e484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. Fabiani R, Minelli L, Bertarelli G, et al. A Western Dietary Pattern Increases Prostate Cancer Risk: A Systematic Review and Meta-Analysis. Nutrients 2016;8:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80]. McGuire S. World Cancer Report 2014. Geneva, Switzerland: World Health Organization, International Agency for Research on Cancer, WHO Press, 2015. Adv Nutr 2016;7:418–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81]. Zhang Y, Porter AL, Hu ZY, et al. Term clumping” for technical intelligence: A case study on dye-sensitized solar cells. Technol Forecast Soc 2014;85:26–39. [Google Scholar]
- [82]. Glanzel W. National characteristics in international scientific co-authorship relations. Scientometrics 2001;51:69–115. [Google Scholar]
- [83]. Tahamtan I, Afshar AS, Ahamdzadeh K. Factors affecting number of citations: a comprehensive review of the literature. Scientometrics 2016;107:1195–225. [Google Scholar]
- [84]. Franceschet M, Costantini A. The effect of scholar collaboration on impact and quality of academic papers. J Informetr 2010;4:540–53. [Google Scholar]
- [85]. Chen HQ, Wang XP, He L, et al. Chinese energy and fuels research priorities and trend: A bibliometric analysis. Renew Sust Energ Rev 2016;58:966–75. [Google Scholar]
- [86]. Acosta M, Coronado D, Ferrandiz E, et al. Regional Scientific Production and Specialization in Europe: The Role of HERD. Eur Plan Stud 2014;22:949–74. [Google Scholar]
- [87]. Ebadi A, Schiffauerova A. How to boost scientific production? A statistical analysis of research funding and other influencing factors. Scientometrics 2016;106:1093–116. [Google Scholar]
- [88]. Nag S, Yang H, Buccola S, et al. Productivity and financial support in academic bioscience. Appl Econ 2013;45:2817–26. [Google Scholar]
- [89]. Read KB, Sheehan JR, Huerta MF, et al. Sizing the Problem of Improving Discovery and Access to NIH-Funded Data: A Preliminary Study. PLoS One 2015;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90]. The Prostate Cancer Foundation Announces Recipients of the 2018 PCF Young Investigator Awards 2018. [Google Scholar]
- [91]. China CoNNSFo. The statistics of projects funded by National Natural Science Foundation of China in 2018. http://www.nsfc.gov.cn/publish/portal0/tab505/, 2018. [Google Scholar]
- [92]. DeAngelis CD. The influence of money on medical science. JAMA 2006;296:996–8. [DOI] [PubMed] [Google Scholar]
- [93]. Moses H, Dorsey ER, Matheson DHM, et al. Financial anatomy of biomedical research. Jama-J Am Med Assoc 2005;294:1333–42. [DOI] [PubMed] [Google Scholar]
- [94]. Potter GA, Hardcastle IR, Jarman M. A convenient, large-scale synthesis of abiraterone acetate [3 beta-acetoxy-17-(3-pyridyl)androsta-5,16-diene], a potential new drug for the treatment of prostate cancer. Org Prep Proced Int 1997;29:123–8. [Google Scholar]
- [95]. Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research - A systematic review. Jama-J Am Med Assoc 2003;289:454–65. [DOI] [PubMed] [Google Scholar]
- [96]. Lexchin J, Bero LA, Djulbegovic B, et al. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ-British Medical Journal 2003;326:1167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97]. Lundh A, Lexchin J, Mintzes B, et al. Industry sponsorship and research outcome. Cochrane Database Syst Rev 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98]. Flacco ME, Manzoli L, Boccia S, et al. Head-to-head randomized trials are mostly industry sponsored and almost always favor the industry sponsor. J Clin Epidemiol 2015;68:811–20. [DOI] [PubMed] [Google Scholar]
- [99]. Rothman DJ, McDonald WJ, Berkowitz CD, et al. Professional Medical Associations and Their Relationships With Industry A Proposal for Controlling Conflict of Interest. Jama-J Am Med Assoc 2009;301:1367–72. [DOI] [PubMed] [Google Scholar]
- [100]. Kahn JO, Cherng DW, Mayer K, et al. Evaluation of HIV-1 immunogen, an immunologic modifier, administered to patients infected with HIV having 300 to 549 x 10(6)/L CD4 cell counts - A randomized controlled trial. Jama-J Am Med Assoc 2000;284:2193–202. [DOI] [PubMed] [Google Scholar]
- [101]. Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis - The CLASS study: A randomized controlled trial. Jama-J Am Med Assoc 2000;284:1247–55. [DOI] [PubMed] [Google Scholar]
- [102]. Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med 2000;343:1520–8. [DOI] [PubMed] [Google Scholar]
- [103]. Curfman GD, Morrissey S, Drazen JM. Expression of concern reaffirmed. N Engl J Med 2006;354:1193–293. [DOI] [PubMed] [Google Scholar]
- [104]. Gibson L. GlaxoSmidiKline to publish clinical trials after US lawsuit. Brit Med J 2004;328:1513–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105]. Ross JS, Hill KP, Egilman DS, et al. Guest authorship and ghostwriting in publications related to rofecoxib - A case study of industry documents from rofecoxib litigation. Jama-J Am Med Assoc 2008;299:1800–12. [DOI] [PubMed] [Google Scholar]
- [106]. Chen K, Yao Q, Sun J, et al. International publication trends and collaboration performance of China in healthcare science and services research. Isr J Health Policy Res 2016;5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Wu YQ, Wan YH, Zhang FZ. Characteristics and Trends of C-H Activation Research: A Review of Literature. Curr Org Synth 2018;15:781–92. [Google Scholar]
- [108]. Bao G, Fang H, Chen L, et al. Soft Robotics: Academic Insights and Perspectives Through Bibliometric Analysis. Soft Robot 2018;5:229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109]. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502–12. [DOI] [PubMed] [Google Scholar]
- [110]. Shore ND. Darolutamide (ODM-201) for the treatment of prostate cancer. Expert Opin Pharmacother 2017;18:945–52. [DOI] [PubMed] [Google Scholar]
- [111]. Rathkopf DE, Antonarakis ES, Shore ND, et al. Safety and Antitumor Activity of Apalutamide (ARN-509) in Metastatic Castration-Resistant Prostate Cancer with and without Prior Abiraterone Acetate and Prednisone. Clin Cancer Res 2017;23:3544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112]. Porter AL, Garner J, Newman NC, et al. National nanotechnology research prominence. Technol Anal Strateg 2019;31:25–39. [Google Scholar]
- [113]. Carley SF, Newman NC, Porter AL, et al. An indicator of technical emergence. Scientometrics 2018;115:35–49. [Google Scholar]
- [114]. Garner J, Carley S, Porter AL, et al. Technological Emergence Indicators Using Emergence Scoring. Portl Int Conf Manag 2017. [Google Scholar]
- [115]. Zechmann CM, Afshar-Oromieh A, Armor T, et al. Radiation dosimetry and first therapy results with a I-124/I-131-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging 2014;41:1280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116]. Tagawa ST, Milowsky MI, Morris M, et al. Phase II Study of Lutetium-177-Labeled Anti-Prostate-Specific Membrane Antigen Monoclonal Antibody J591 for Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res 2013;19:5182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117]. Weineisen M, Schottelius M, Simecek J, et al. Ga-68- and Lu-177-Labeled PSMA I&T: Optimization of a PSMA-Targeted Theranostic Concept and First Proof-of-Concept Human Studies. J Nucl Med 2015;56:1169–76. [DOI] [PubMed] [Google Scholar]
- [118]. Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha-Therapy of Metastatic Castration-Resistant Prostate Cancer with Ac-225-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J Nucl Med 2017;58:1624–31. [DOI] [PubMed] [Google Scholar]
- [119]. Yordanova A, Becker A, Eppard E, et al. The impact of repeated cycles of radioligand therapy using [Lu-177] Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur J Nucl Med Mol Imaging 2017;44:1473–9. [DOI] [PubMed] [Google Scholar]
- [120]. Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German Multicenter Study Investigating Lu-177-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J Nucl Med 2017;58:85–90. [DOI] [PubMed] [Google Scholar]
- [121]. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer 2017;17:223–38. [DOI] [PubMed] [Google Scholar]
- [122]. Siravegna G, Marsoni S, Siena S, et al. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 2017;14:531–48. [DOI] [PubMed] [Google Scholar]
- [123]. Annala M, Vandekerkhove G, Khalaf D, et al. Circulating Tumor DNA Genomics Correlate with Resistance to Abiraterone and Enzalutamide in Prostate Cancer. Cancer Discov 2018;8:444–57. [DOI] [PubMed] [Google Scholar]
- [124]. Mateo J, Boysen G, Barbieri CE, et al. DNA Repair in Prostate Cancer: Biology and Clinical Implications. Eur Urol 2017;71:417–25. [DOI] [PubMed] [Google Scholar]
- [125]. Cheng HH, Pritchard CC, Boyd T, et al. Biallelic Inactivation of BRCA2 in Platinum-sensitive Metastatic Castration-resistant Prostate Cancer. Eur Urol 2016;69:992–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Giacinti SPG, Roberto M, Macrini S, et al. Molecular Basis of Drug Resistance and Insights for New Treatment Approaches in mCRPC. Anticancer Res 2018;38:6029-6039. [DOI] [PubMed] [Google Scholar]
- [127]. van Soest RJ, van Royen ME, de Morree ES, et al. Cross-resistance between taxanes and new hormonal agents abiraterone and enzalutamide may affect drug sequence choices in metastatic castration-resistant prostate cancer. Eur J Cancer 2013;49:3821–30. [DOI] [PubMed] [Google Scholar]


