Abstract
This study aimed to identify the correlation of contradiction between DAPT score and PRECISE-DAPT score with the severity of coronary lesion in acute coronary syndromes (ACS).
In total, 458 patients with ACS after a percutaneous coronary intervention (PCI) who had tolerated 1-year uneventful dual antiplatelet therapy (DAPT) were enrolled and divided into groups based on the Gensini score, number of stenosed vessels, and left main (LM) disease. Both DAPT score and PRECISE-DAPT score were calculated and the proportion of patients receiving conflicting recommendations from each score was compared among the groups.
DAPT score as well as the proportion of patients with DAPT score ≥2 were associated with the Gensini score and the number of stenosed vessels. Similarly, PRECISE-DAPT score as well as the proportion of patients with PRECISE-DAPT score ≥25 were associated with the Gensini score and the number of stenosed vessels. The proportion of patients with DAPT score ≥2 along with PRECISE-DAPT score ≥25 were associated with Gensini score, but they had no significant association with the number of stenosed vessels (P = .006 and P = .075, respectively). None of those aforementioned items were associated with LM disease.
The inconsistencies of DAPT scores and PRECISE-DAPT scores are frequent and associated with the severity of coronary disease, represented by the Gensini score. Appropriate clinical decisions should be individualized.
Keywords: acute coronary syndrome, DAPT score, percutaneous coronary intervention, PRECISE-DAPT score
1. Introduction
Dual antiplatelet therapy (DAPT), a combination of aspirin with a P2Y12 inhibitor, remains a sought-after research area due to the increasing population with acute coronary syndromes (ACS) undergoing percutaneous coronary intervention (PCI). DAPT is used to protect stented vessels from thrombosis and avoid other major adverse cardiac and cerebral events (MACCE). Nonetheless, this antithrombotic protection is counterbalanced by the simultaneous pro-hemorrhagic effects, making the exact duration of DAPT still a matter of debate.
New guidelines for DAPT in ACS patients after PCI have been proposed in the recent years.[1–3] It is of note that decisions about DAPT duration mainly depend on the patients’ risk stratification, which should be pivotal before the treatment. Based on this consideration, quantitative risk scores have been developed to help estimate ischemic or bleeding risk. It is challenging, however, to appraise both risks simultaneously because of overlapping risk factors. Novel scoring systems might prove useful to tailor DAPT regimens to maximize the benefits with minimal associated harms.[3]
There are 2 new developed risk prediction models for decision-making surrounding the optimal duration of DAPT, namely DAPT score and PRECISE-DAPT (PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent DAPT) score. In 2017, the European Society of Cardiology (ESC) updated a guideline to recommend the use of them for the first time.[4,5] Both scores have been validated since then. It was also revealed in our previous study that DAPT score and PRECISE-DAPT score were independently associated with the degree of coronary stenosis in patients with ACS.[6] It is plausible that patients with more severe coronary lesions obtain higher points in both scoring systems, and thus get divergent suggestions on DAPT duration. Given the valuable need to make an optimal clinical decision for such patients, the present study aims to discover the correlation of contradiction between DAPT score and PRECISE-DAPT score with the severity of coronary lesion in patients with ACS, which has been neglected.
2. Methods
2.1. Patients
The present retrospective, observational study included a cohort of consecutive patients who were diagnosed with ACS and had undergone primary or elective PCI with drug-eluting stents (DES) at the Department of Cardiology, Xiangya Hospital of Central South University, Changsha, China from January 2012 to December 2013. Patients with atrial fibrillation (AF), severe hepatic or renal insufficiency, infections, inflammatory conditions, malignancies, or those with incomplete clinical data were excluded. The ACS, including non-ST-elevation ACS and ST-elevation myocardial infarction (STEMI), was diagnosed according to the American Heart Association/American College of Cardiology (AHA/ACC) and the ESC guidelines.[7,8] All participants had taken DAPT after PCI for at least 1 year and were not on chronic oral anticoagulation. Baseline information was determined from the electronic medical record, and telephone interviews were conducted monthly to ascertain whether DAPT had been taken as planned and ensure no adverse events had happened in the first year. In total, 458 patients were included in our study. The study was approved by the Ethics Committee of our hospital, and signed informed consent was obtained from all patients.
2.2. Auxiliary examination
Blood samples were collected on the morning following admission after an overnight fast. They were subsequently analyzed for parameters including white blood cell (WBC) count, hemoglobin and creatinine levels, and lipid profiles (Roche Diagnostics GmbH, Mannheim, Germany). Creatinine clearance (CrCl) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.[9] Transthoracic echocardiography was performed using a Hewlett-Packard Sonos 1000 ultrasound system (Hewlett-Packard, Palo Alto, CA) during hospitalization, and left ventricular ejection fraction (LVEF) was calculated by the Simpson method.[10]
2.3. Risk scores calculation
DAPT score ranging from −2 to 10 points was calculated for each patient. In detail, −2 points were given for age ≥75 years, while −1 point for age between 65 and 75 years, 0 point for age <65 years, 1 point each for cigarette smoking, diabetes mellitus, myocardial infarction (MI) at presentation, prior PCI or prior MI, paclitaxel-eluting stent and stent diameter <3 mm, and 2 points for vein graft stent and congestive heart failure (CHF) or LVEF <30%, respectively. The total score was determined by summation of all points of the 9 predictive risk factors. Likewise, PRECISE-DAPT score ranging from 0 to 100 points was calculated with a score nomogram. There were 5 predictive risk factors, namely age, CrCl, hemoglobin level, WBC count, and history of previous spontaneous bleeding, each assigned a corresponding value on the point axis based on their levels, and the summation of those points determined the total score. Both scores were obtained via a free calculator available in Apple's App Store (American College of Cardiology for DAPT Risk Calculator, version 1.5.1 and Alma Digit Srl for Precise DAPT, version 1.2). Long DAPT (30 months) is recommended for patients with a DAPT score ≥2 due to higher ischemic risks, and in contrast, short DAPT (3–6 months) is recommended for patients with a PRECISE-DAPT score ≥25 because of higher bleeding risks.
2.4. Coronary stenosis assessment
The severity of coronary stenosis was independently assessed by Gensini score[11] by 2 experienced interventional cardiologists blinded to our study while coronary angiography was performed. Specifically, scores of 1, 2, 4, 8, 16, and 32 points were used to indicate the stenosis of 1% to 25%, 26% to 50%, 51% to 75%, 76% to 90%, 91% to 99%, and 100%. Additionally, a multiplying factor representing the functional significance of the area supplied by each vascular segment was given. For example, a factor of 0.5 was given to the second diagonal branch; a factor of 1 was given to the first diagonal branch, the distal segment of left anterior descending (LAD), the obtuse marginal branch, the proximal, middle or distal segment of the right coronary artery (RCA), the posterior descending artery, the distal segment of circumflex (LCX), and left ventricular posterior branch; a factor of 1.5 was given to the middle segment of LAD; a factor of 2.5 was given to the proximal LAD or LCX; and a factor of 5 was given to the left main (LM). The stenosis of each segment was described by multiplication of the factor and the corresponding scores, which was summed to obtain the total Gensini score.[12] Significant artery stenosis was defined if ≥50% of the lumen diameter was reduced in any vessel. Stenosis of diagonal branch, obtuse marginal branch, and left ventricular posterior branch was regarded as stenosis of LAD, LCX, and RCA, respectively, and LM disease was recorded as stenosis of LAD and LCX.[13] The number, total length, and minimum diameter of stent were also collected.
2.5. Statistical analysis
Kolmogorov–Smirnov test was used to analyze data normality. Continuous variables were presented as mean ± standard deviation or median (interquartile range) as appropriate, and compared using t test, Mann–Whitney U test or Kruskal–Wallis H test according to distribution characteristics and homoscedasticity of data. Categorical variables were expressed as percentages and compared using Χ2 test or Fisher's exact test. Bland–Altman method was used to analyze the reproducibility of interventional cardiologists and intraobserver as well as interobserver variabilities were expressed as the 95% confidence interval for relative differences. A two-sided P value < .05 was considered statistically significant. The statistical analysis was conducted by IBM SPSS Statistics version 22.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Baseline characteristics
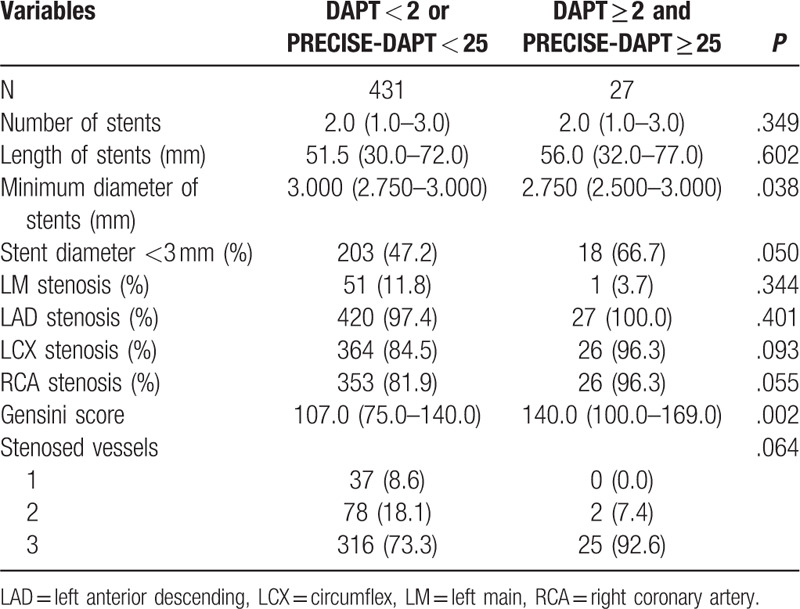
A total of 458 consecutive ACS patients undergoing DES implantation were included in our final analysis and divided into 2 groups based on the combination of DAPT score and PRECISE-DAPT score. Among them, 27 (5.9%) patients with DAPT score ≥2 and PRECISE-DAPT score ≥25 were assigned to the conflicting group, while the others constituted another group. Neither ischemic nor bleeding events that might terminate DAPT were observed during the first year after index PCI. The intraobserver variabilities of Gensini scores were −0.84% to 1.19% and −1.71% to 0.76%, while the interobserver variability of Gensini scores was −1.37% to 2.23%. The baseline demographic, laboratorial, angiographic, and procedural characteristics of the entire population are summarized (Tables 1 and 2).
Table 1.
The baseline demographic and laboratorial characteristics of study population.
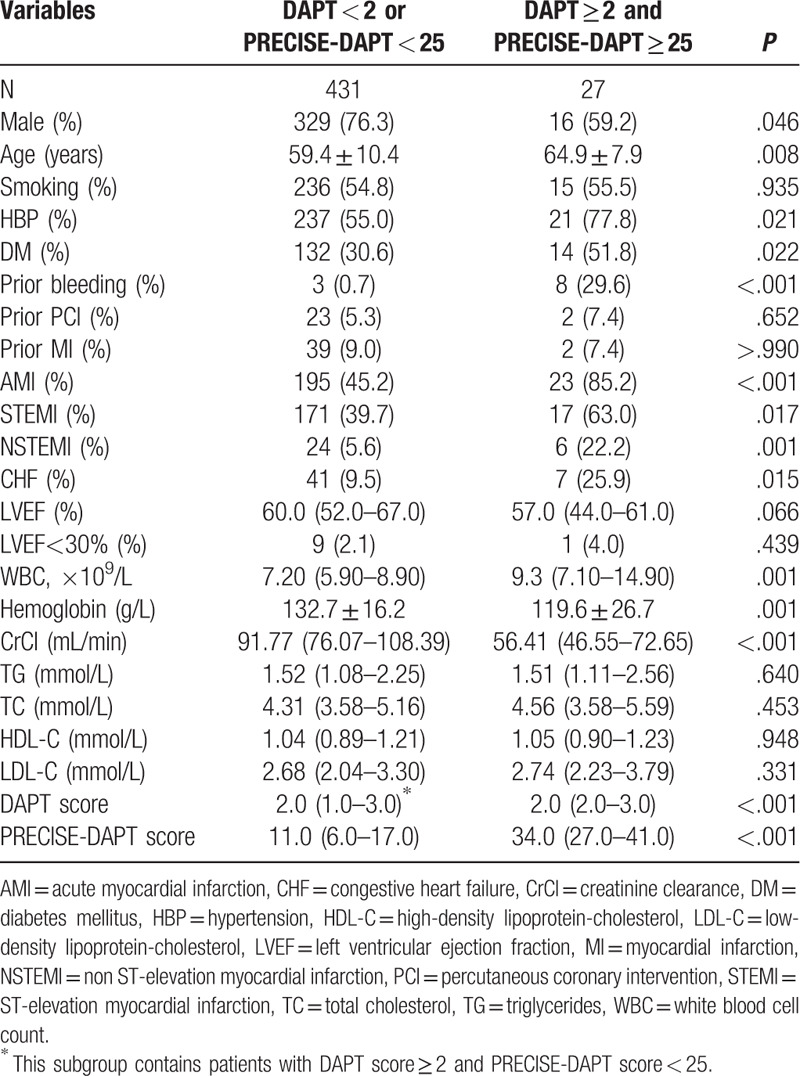
Table 2.
The baseline angiographic and procedural characteristics of study population.
Patients in the conflicting group were significantly older, more frequently female, more likely to have a history of hypertension or diabetes mellitus, prior bleeding, presented more often with acute MI or CHF. However, cigarette smoking history, prior PCI, or prior MI was similar between groups.
In terms of accessory examinations, patients in conflicting group had higher WBC counts and lower hemoglobin or CrCl levels. Besides, there was no statistical difference regarding lipid profiles, LVEF or proportions of patients with LVEF<30%. Both DAPT score and PRECISE-DAPT score were higher in conflicting group as well.
In addition, patients in the conflicting group also displayed a higher Gensini score and were implanted with stents of smaller minimum diameters. Meanwhile, no significant differences were detected with respect to number or length of stents, proportions of stent diameter <3 mm, percentages of each coronary stenoses, and number of stenosed vessels. Furthermore, neither paclitaxel-eluting stents nor vein graft stents were implanted in any of the patients analyzed in this study.
3.2. Contradictions according to Gensini score
The study population was also divided in tertiles by Gensini score (Table 3 and Fig. 1). In detail, 3 groups were described as follows: T1: 12 to 89 points, T2: 90 to 128 points, and T3: 129 to 316 points. Both DAPT score and PRECISE-DAPT score increased with increasing tertiles of Gensini score (P < .001 and P = .001, respectively). Meanwhile, proportions of patients with DAPT score ≥2 or PRECISE-DAPT score ≥25 met the same trend (P < .001 and P = .006, respectively). When both scores were combined, proportions of patients with conflicting recommendations showed significant difference among groups, in which a higher proportion had a higher Gensini score; the highest proportion was found in the third tertile (P = .006).
Table 3.
Contradictions according to Gensini score.
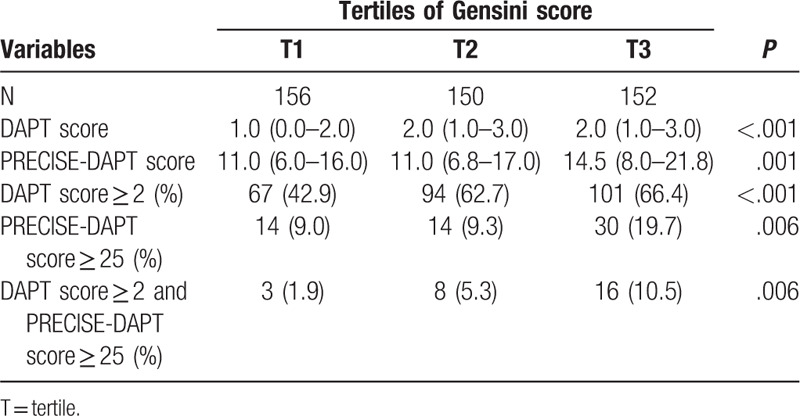
Figure 1.
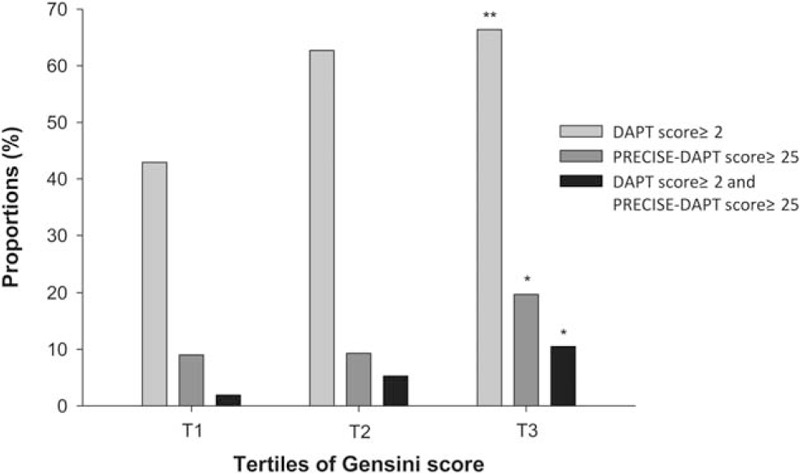
Contradictions according to Gensini score. Proportions of patients with DAPT score ≥2, PRECISE-DAPT score ≥25, and DAPT score ≥2 while PRECISE-DAPT score ≥25 were associated with Gensini score. ∗P < .01 among tertiles of Gensini score. ∗∗P < .001 among tertiles of Gensini score.
3.3. Contradictions according to stenosed vessels
Next, patients were classified based on stenosed vessels (Table 4 and Fig. 2). More stenosed vessels resulted in a higher DAPT score and PRECISE-DAPT score (P < .001, for all). Proportions of patients with DAPT score ≥2 or PRECISE-DAPT score ≥25 also showed a similar trend with an increase in the number of stenosed vessels (P = .002 and P = .041, respectively). No significant difference was observed in the proportions of patients with conflicting recommendations (P = .075).
Table 4.
Contradictions according to stenosed vessels.
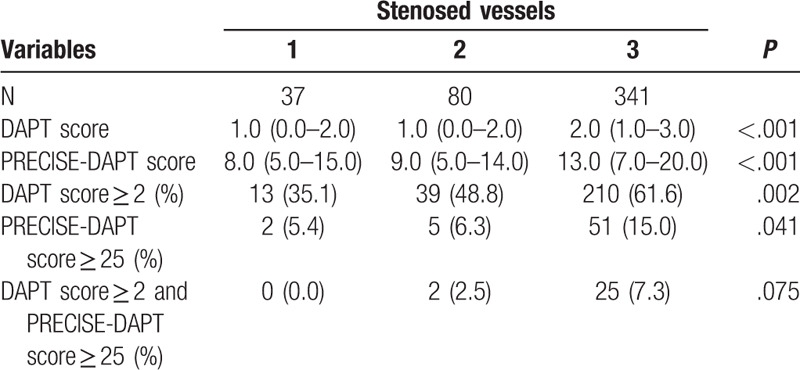
Figure 2.
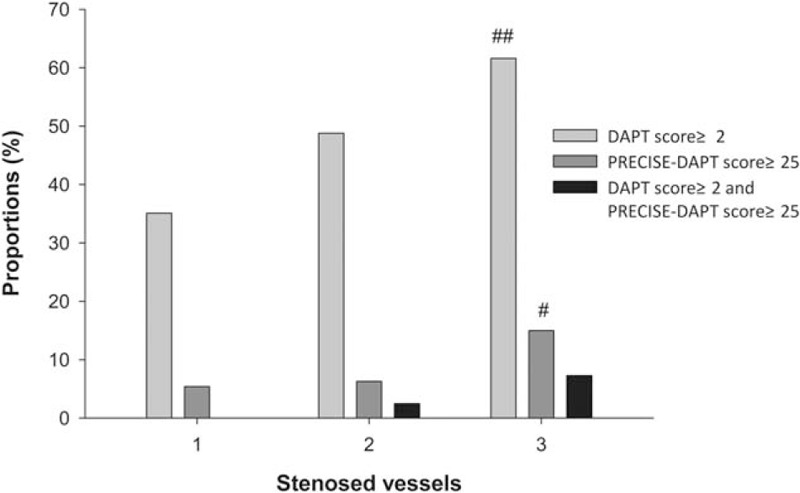
Contradictions according to stenosed vessels. Proportions of patients with DAPT score ≥2 and PRECISE-DAPT score ≥25 were associated with number of stenosed vessels, but proportions of patients with DAPT score ≥2 while PRECISE-DAPT score ≥25 had no significant association with number of stenosed vessels despite tendency of positive correlation. # P < .05 among subgroups of stenosed vessels. ## P < .01 among subgroups of stenosed vessels.
3.4. Contradictions according to LM disease
Finally, we assigned patients with or without LM disease to corresponding groups (Table 5 and Fig. 3). There was no statistical difference in terms of DAPT score, PRECISE-DAPT score, as well as proportions of patients with DAPT score ≥2, PRECISE-DAPT score ≥25, and DAPT score ≥2 while PRECISE-DAPT score ≥25 between groups (P > .05, for all).
Table 5.
Contradictions according to left main disease.
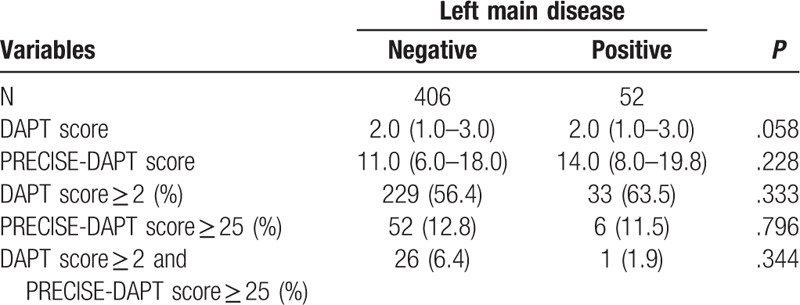
Figure 3.
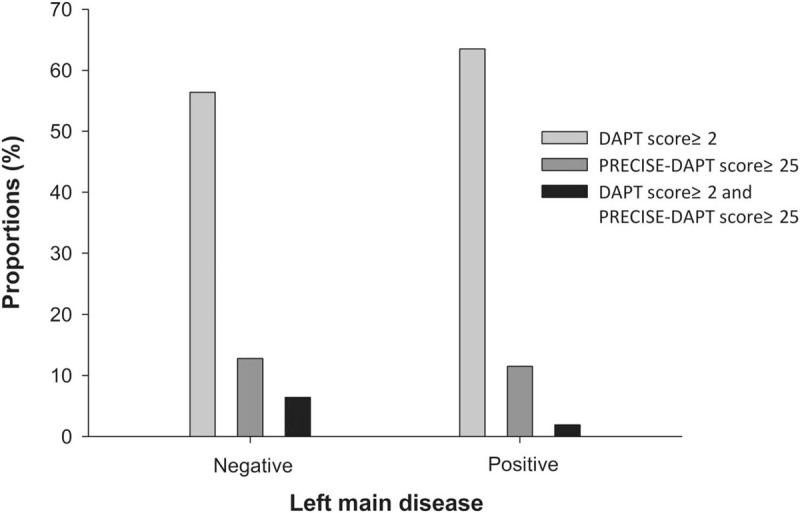
Contradictions according to LM disease. No proportion of patients with DAPT score ≥2, PRECISE-DAPT score ≥25, or DAPT score ≥2 while PRECISE-DAPT score ≥25 were associated with left main disease.
4. Discussion
This is the first study evaluating the association between the inconsistency of the 2 novel DAPT decision-making tools and the severity of coronary lesions in patients with ACS receiving stents placement. Our results revealed that DAPT score and the proportion of patients with DAPT score ≥2 as well as PRECISE-DAPT score and the proportions of patients with PRECISE-DAPT score ≥25 were associated with Gensini score and number of stenosed vessels. In addition, the proportion of patients with DAPT score ≥2 while PRECISE-DAPT score ≥25 were associated with the Gensini score, but it had no significant association with number of stenosed vessels despite a tendency of positive correlation. None of aforementioned items, however, were associated with LM disease.
It is known that ACS is the leading causes of morbidity and mortality in the world and causes great economic burden to societies.[14,15] With the advancement of reperfusion and optimization of pharmacotherapy, the prognosis of ACS patients has improved.[16] As a cornerstone treatment, DAPT remains a highly effective way to mitigate ischemic events across the board, albeit with concomitant risk of bleeding episodes. Therefore, it is of paramount importance to evaluate the subtle balance between the benefits and harms in order to tailor optimal DAPT regimens for individual patients. Contemporarily, risk stratification tools have been developed to guide clinical decisions. Residual SYNTAX score, an index to assess completion of PCI, has been demonstrated as a strong independent predictor of ischemic events.[17] HAS-BLED score, utilized to predict bleeding events in patients with AF, has been recently shown to estimate the incidence of major bleeding and death in patients with DES deployment regardless of AF.[18,19] Although both provide advice to some extent, neither of them can simultaneously appraise the benefits and harms of DAPT or were widely used.
Recently, DAPT score and PRECISE-DAPT score were proposed and both have been included in the 2017 ESC Guideline as a Class IIb recommendation.[1,3] Specifically, long DAPT (30 months) can be considered for patients with a DAPT score ≥2, whereas standard DAPT (12 months) may be recommendable for those with a DAPT score <2. Besides, short DAPT (3–6 months) is recommended for patients with a PRECISE-DAPT score ≥25, while standard/long DAPT (12–24 months) may be favorable for those with a PRECISE-DAPT score <25. Once developed, both scores have been validated with differing results outside the original studies. In a pooled cohort of 12,223 patients from Japan, DAPT score was demonstrated as a robust prediction performance score system for ischemic and bleeding risks beyond 1 year after PCI with DES.[20] In a Chinese study involving 6088 ACS patients treated with DES, DAPT score did not predict long-term ischemic events well, although it showed a modest capacity for prediction of later major bleeding.[21] In contrast, DAPT score failed to adequately discriminate ischemic and bleeding risks over 1 year in a Swedish study covering 41,101 patients after stenting from the real world.[22] Despite being controversial, such scores may play promising roles in individualizing DAPT duration with a single scoring system.[23] Besides, we had also revealed previously that both DAPT score and PRECISE-DAPT score were independently and positively correlated with Gensini score and risk of triple-vessel disease.[6]
Moving forward, the present study showed that higher scores and proportions of patients with scores over cut-offs were related to higher Gensini scores or more stenosed vessels in both scoring systems. It is known that a higher DAPT score and PRECISE-DAPT score denote higher ischemic and bleeding risk, respectively, inferring that patients with more severe coronary lesions might have higher risks in both and would have a DAPT score ≥2 and PRECISE-DAPT score ≥25 at the same time, and thus receiving conflicting recommendations. There were 5.9% patients in the present study obtaining conflicting recommendations who seemed to present worse baseline characteristics. Further analysis suggested that such contradictory recommendations were positively correlated to the Gensini score. This suggests that increasingly serious lesions in the minority of more severe patients results in more prominent contradictions. Hence, it is challenging to determine an optimal duration of DAPT for severe patients. This subgroup of patients may have more advanced disease with a higher prevalence of comorbidities and are more prone to natural progression and acute changes of coronary lesions. They may undergo more complex PCI with more or longer stents and, thus, have an increased likelihood of incomplete revascularization and additional potential sites of delayed endothelialization, both of which could trigger stent thrombosis.[24] Pooled data from 6 randomized controlled trials (RCTs) suggested a DAPT duration ≥1 year than a 3- to 6-month duration in patients with complex PCI due to greater antithrombotic protection alongside a similar risk of bleeding. The benefits of prolonged DAPT progressively increased with the degree of procedural complexity.[24] Post hoc analysis of the DAPT Study subsequently demonstrated that the benefits of prolonged DAPT were associated with anatomic complexity of target lesion, particularly within the first year after index procedure, but this association was attenuated thereafter.[25] However, an umbrella review summarized that the risk of MACCE was similar between prolonged and abbreviated DAPT among patients with and without complex lesions.[26] Collectively, these findings indicate that patient complexity, as evaluated by DAPT score, is more important than lesion complexity in decision-making on continuation of DAPT for those reaching 1 year uneventfully and that patients of high DAPT scores with either complex or simple lesions may benefit the most from extending DAPT.[25,27] With respect to lesion complexity, it may just determine who might benefit from continued DAPT beyond a minimum period of 3 or 6 months, which approximately coincides with the time for vascular healing and platform endothelialization with new generation DES.[24,25] These findings may be explained by the fact that benefits of DAPT extend beyond target vascular segment to entire coronary tree and even the systemic arteries.[24,27] As such, complexity of index lesion may not be an adequate predictor of a patient's future ischemic risk.[25,27] It is of note that DAPT, with its systemic effect, exerts antithrombotic protection at the expense of increased bleeding risk, irrespective of procedural complexity.[24] A German study with 3976 patients, treated with second-generation DES from the Intracoronary Stenting and Antithrombotic Regimen: Safety and Efficacy of 6 Months DAPT After Drug-Eluting Stenting (ISAR-SAFE) trial showed no significant differences in ischemic outcomes between 6 and 12 months DAPT in those with high DAPT scores.[28] The 6 Months Versus 12 Months DAPT After DES in ST-elevation Myocardial Infarction (DAPT-STEMI) trial involving 870 patients from 17 European study sites additionally showed that a DAPT for 6 months was non-inferior to that for 1 year in patients with uneventful STEMI at 6 months after primary PCI with second-generation DES.[29] The above result was extended to patients with ACS, the majority of whom were treated with second-generation DES in a systematic review and meta-analysis of 10 RCTs.[30] It seems reasonable to hypothesize a curtailment of ischemic effect from lesion complexity since the advent of second-generation DES.[27] Based on these evidences, the exact DAPT duration for those severe patients remains questionable. To focus on the inconsonant conditions, we proposed a step-by-step solution to aid decision-making of DAPT duration for severe patients, especially for those with high Gensini scores.[6] In detail, PRECISE-DAPT score was calculated at the time of index PCI with new generation DES to decide a short or standard DAPT. If a standard regimen was conducted uneventfully to 1 year, DAPT score was further calculated to determine whether to extend DAPT duration beyond 1 year. Due to constant contradictions of ischemic and bleeding risks, close follow-ups should be conducted in severe patients even if recommended therapies were accomplished. However, this method had not yet been validated. It should be noted that all risk scores are complements rather than substitutes for clinical judgment[2] and decisions should be individualized.
This study has some limitations. First, it is a retrospective analysis with a small study population in a single center. Second, the dominant pattern of coronary artery was not taken into account. Additionally, we did not thoroughly analyze potential effects on adverse risks from any significant stenoses that had not been stented. Furthermore, diversities of types or dosages of P2Y12 inhibitors used in our study were not considered. Meanwhile, neither paclitaxel-eluting stents nor vein graft stents were implanted in any patients, resulting in somewhat lower DAPT scores. Finally, 5.9% subjects constituted to the conflicting group, making a rather heterogenous distribution. To better evaluate, the utility of both scores in patients with serious diseases, further studies would be warranted.
In conclusion, this is the first study of its kind and reveals that the inconsistencies of DAPT and PRECISE-DAPT scores are frequent and associated with the severity of coronary disease, represented by the Gensini score. Appropriate clinical decisions should be individualized.
Acknowledgments
We are very grateful for the assistance from the Xiangya School of Public Health for statistical analysis.
Author contributions
Conceptualization: Chenglong Zhang.
Data curation: Sisi Bi, Yue Zhao.
Formal analysis: Sisi Bi.
Investigation: Yue Zhao, Qingling Peng, Wenxue Liu.
Methodology: Sisi Bi, Chenglong Zhang.
Project administration: Guogang Zhang.
Resources: Guogang Zhang, Chenglong Zhang.
Software: Sisi Bi, Yue Zhao.
Supervision: Guogang Zhang, Chenglong Zhang.
Writing – review & editing: Sisi Bi, Chenglong Zhang.
Footnotes
Abbreviations: ACS = acute coronary syndrome, AF = atrial fibrillation, CHF = congestive heart failure, CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration, CrCl = creatinine clearance, DAPT = dual antiplatelet therapy, DES = drug-eluting stent, LAD = left anterior descending, LCX = circumflex, LM = left main, LVEF = left ventricular ejection fraction, MACCE = major adverse cardiac and cerebral events, MI = myocardial infarction, PCI = percutaneous coronary intervention, RCA = right coronary artery, RCT = randomized controlled trials, STEMI = ST-elevation myocardial infarction, WBC = white blood cell.
How to cite this article: Bi S, Zhao Y, Peng Q, Liu W, Zhang G, Zhang C. Contradictions between DAPT and PRECISE-DAPT scores with the severity of coronary lesion in acute coronary syndrome. Medicine. 2020;99:16(e19699).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68:1082–115. [DOI] [PubMed] [Google Scholar]
- [2].Mehta SR, Bainey KR, Cantor WJ, et al. 2018 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol 2018;34:214–33. [DOI] [PubMed] [Google Scholar]
- [3].Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the task force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018;39:213–60. [DOI] [PubMed] [Google Scholar]
- [4].Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 2017;389:1025–34. [DOI] [PubMed] [Google Scholar]
- [5].Yeh RW, Secemsky EA, Kereiakes DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA 2016;315:1735–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Long T, Peng L, Li F, et al. Correlations of DAPT score and PRECISE-DAPT score with the extent of coronary stenosis in acute coronary syndrome. Medicine (Baltimore) 2018;97:e12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–228. [DOI] [PubMed] [Google Scholar]
- [8].Ibanez B, James S, Agewall S, et al. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–77. [DOI] [PubMed] [Google Scholar]
- [9].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63. [DOI] [PubMed] [Google Scholar]
- [11].Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol 1983;51:606. [DOI] [PubMed] [Google Scholar]
- [12].Ma CY, Xu ZY, Wang SP, et al. Change of inflammatory factors in patients with acute coronary syndrome. Chin Med J (Engl) 2018;131:1444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang C, Liu P, Xia K, et al. Association of serum prealbumin with angiographic severity in patients with acute coronary syndrome. Med Sci Monit 2017;23:4041–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huang YJ, Parry M, Zeng Y, et al. Examination of a nurse-led community-based education and coaching intervention for coronary heart disease high-risk individuals in China. Asian Nurs Res (Korean Soc Nurs Sci) 2017;11:187–93. [DOI] [PubMed] [Google Scholar]
- [15].Sayed AS, Xia K, Salma U, et al. Diagnosis, prognosis and therapeutic role of circulating miRNAs in cardiovascular diseases. Heart Lung Circ 2014;23:503–10. [DOI] [PubMed] [Google Scholar]
- [16].Zhang C, Li F, Long T, et al. Beta 2-microglobulin and the severity of coronary stenosis in patients with acute coronary syndrome. Heart Lung Circ 2018;28:575–82. [DOI] [PubMed] [Google Scholar]
- [17].Qiu M, Li Y, Li J, et al. Impact of six versus 12 months of dual antiplatelet therapy in patients with drug-eluting stent implantation after risk stratification with the residual SYNTAX score: results from a secondary analysis of the I-LOVE-IT 2 trial. Catheter Cardiovasc Interv 2017;89(S1):565–73. [DOI] [PubMed] [Google Scholar]
- [18].Konishi H, Miyauchi K, Tsuboi S, et al. Impact of the HAS-BLED score on long-term outcomes after percutaneous coronary intervention. Am J Cardiol 2015;116:527–31. [DOI] [PubMed] [Google Scholar]
- [19].Shah RR, Pillai A, Omar A, et al. Utility of the HAS-BLED score in risk stratifying patients on dual antiplatelet therapy post 12 months after drug-eluting stent placement. Catheter Cardiovasc Interv 2017;89:E99–103. [DOI] [PubMed] [Google Scholar]
- [20].Yoshikawa Y, Shiomi H, Watanabe H, et al. Validating utility of dual antiplatelet therapy score in a large pooled cohort from 3 Japanese percutaneous coronary intervention studies. Circulation 2018;137:551–62. [DOI] [PubMed] [Google Scholar]
- [21].Song L, Guan C, Yan H, et al. Validation of contemporary risk scores in predicting coronary thrombotic events and major bleeding in patients with acute coronary syndrome after drug-eluting stent implantations. Catheter Cardiovasc Interv 2018;91(S1):573–81. [DOI] [PubMed] [Google Scholar]
- [22].Ueda P, Jernberg T, James S, et al. External validation of the DAPT score in a nationwide population. J Am Coll Cardiol 2018;72:1069–78. [DOI] [PubMed] [Google Scholar]
- [23].Paravattil B, Elewa H. Strategies to optimize dual antiplatelet therapy after coronary artery stenting in acute coronary syndrome. J Cardiovasc Pharmacol Ther 2017;22:347–55. [DOI] [PubMed] [Google Scholar]
- [24].Giustino G, Chieffo A, Palmerini T, et al. Efficacy and safety of dual antiplatelet therapy after complex PCI. J Am Coll Cardiol 2016;68:1851–64. [DOI] [PubMed] [Google Scholar]
- [25].Yeh RW, Kereiakes DJ, Steg PG, et al. Lesion complexity and outcomes of extended dual antiplatelet therapy after percutaneous coronary intervention. J Am Coll Cardiol 2017;70:2213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wells GA, Elliott J, Kelly S, et al. Dual Antiplatelet Therapy Following Percutaneous Coronary Intervention: A Review of the Clinical Impact of Treatment Duration. Ottawa, ON; 2017. [PubMed] [Google Scholar]
- [27].Colombo A, Giannini F. Long-term duration of dual antiplatelet therapy: patient complexity is more important than lesion complexity. J Am Coll Cardiol 2017;70:2224–5. [DOI] [PubMed] [Google Scholar]
- [28].Harada Y, Michel J, Lohaus R, et al. Validation of the DAPT score in patients randomized to 6 or 12 months clopidogrel after predominantly second-generation drug-eluting stents. Thromb Haemost 2017;117:1989–99. [DOI] [PubMed] [Google Scholar]
- [29].Kedhi E, Fabris E, van der Ent M, et al. Six months versus 12 months dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): randomised, multicentre, non-inferiority trial. BMJ 2018;363:k3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Misumida N, Abo-Aly M, Kim SM, et al. Efficacy and safety of short-term dual antiplatelet therapy (</ = 6 months) after percutaneous coronary intervention for acute coronary syndrome: A systematic review and meta-analysis of randomized controlled trials. Clin Cardiol 2018;41:1455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


