Abstract
Hypothyroidism and chronic kidney disease (CKD) are highly prevalent conditions with a potential mechanistic link. We sought to determine whether hypothyroidism is associated with CKD among a large diverse community-based cohort.
A cross-sectional study was performed (January 1, 1990–December 31, 2017) within a large integrated health system. Individuals age ≥55 years of age with outpatient measurements of thyroid stimulating hormone (TSH) and ≥2 serum creatinine values were included. Hypothyroidism was defined as TSH >4 mIU/L and/or receipt of thyroid hormone replacement and further categorized as hypothyroid status: TSH >4 mcIU/mL and attenuated-hypothyroid status: TSH <4 mcIU/mL with receipt of thyroid hormone replacement. Euthyroidism was defined as TSH <4 mIU/L and no thyroid hormone replacement. Our primary measure was CKD defined as an estimated glomerular filtration rate (eGFR) <45 mL/min/1.73 m2. Multivariable logistic regression adjusting for age, sex, race, and comorbidities was used to estimate odds ratios (OR) for CKD by thyroid status.
Among 378,101 individuals, 114,872 (30.4%) had hypothyroidism among whom 31,242 and 83,630 had hypothyroid and attenuated-hypothyroid statuses, respectively. Individuals with hypothyroidism had a CKD OR (95%CI) of 1.25 (1.21–1.29) compared with those with euthyroidism. Granular examination of thyroid statuses showed that hypothyroid and attenuated-hypothyroid statuses had CKD ORs (95% CI) of 1.59 (1.52–1.66) and 1.12 (1.08–1.16), respectively. A similar relationship was observed in analyses that defined CKD as an eGFR <60 L/min/1.73 m2.
Among individuals 55 years and older, we observed that those with hypothyroidism were more likely to have CKD. A stronger association was found among patients of hypothyroid status compared with attenuated-hypothyroid status suggesting a dose dependent relationship.
Keywords: chronic kidney disease, epidemiology, hypothyroidism
1. Introduction
Chronic kidney disease (CKD) is a highly prevalent condition with numerous health consequences including heightened risk for cardiovascular disease, infections, impaired physical function, and death.[1–3] Approximately 15% of the adult population have CKD, among whom there is a 2-fold higher risk of death and a 1.5-fold higher risk of hospitalization compared with their non-CKD counterparts, even after accounting for differences in socio-demographic characteristics.[1,2] While hypertension and diabetes are well known risk factors, identifying new mechanistic links or risk factors for CKD may impact management strategies.
Thyroid function has been suggested to have a relationship with kidney function and CKD.[4–12] It has been hypothesized that hypothyroidism may lead to altered kidney function via effects on cardiac output, intra-renal hemodynamics, and renin angiotensin aldosterone system (RAAS), as well as structural changes including decreased kidney-to-body weight ratio, truncated tubular mass, and altered glomerular architecture.[13] Patients with CKD have been observed to have a higher prevalence of hypothyroidism.[4–7] Conversely, hypothyroidism has also been suggested to be a risk factor for depressed kidney function.[8–12] Overall, these clinical observations have been limited to select populations.
If hypothyroidism has a mechanistic link to CKD, it may have important clinical implications for the CKD population. Hypothyroidism is not only highly prevalent, but also easily diagnosed and treatable.[14,15] At the same time, there are no uniform screening guidelines for hypothyroidism, particularly in the CKD population.[15–18] In this study, we used electronic health records (EHR) from a large, diverse population of adults age 55 years and older to determine whether hypothyroidism was associated with CKD.
2. Methods
2.1. Source cohort
We performed a cross-sectional study of members within Kaiser Permanente Southern California (KPSC) in the period January 1, 1990 and December 31, 2017.[19] KPSC is an integrated health system providing comprehensive care to over 4.6 million members at 15 medical centers and >200 satellite clinics throughout Southern California. The patient population is racially/ethnically and socio-economically diverse, reflecting the general population of Southern California.[20,21] All KPSC members have similar benefits and access to healthcare services, clinic visits, procedures, and copays for medications. Healthcare encounters are tracked using an EHR from which all study information was extracted. All data for this study were collected as part of routine clinical encounters in which healthcare providers determined the need for laboratory measurements, procedures, and medications. The study protocol was reviewed and approved by the KPSC Institutional Review Board (No. 10758) and was deemed exempt from informed consent.
2.2. Study population
Individuals ≥55 years of age with outpatient measurements of serum TSH and 2 serum creatinine values were included in the study population (Fig. 1). Only outpatient measurements were chosen to minimize risk of misclassification of thyroid status during acute illnesses (i.e., non-thyroidal illness) and to minimize capturing acute kidney injury. Exclusion criteria included individuals with hyperthyroidism, end stage renal disease on renal replacement therapy, polycystic kidney disease, glomerulonephritis, rheumatologic or autoimmune diseases, or prior renal transplant.
Figure 1.
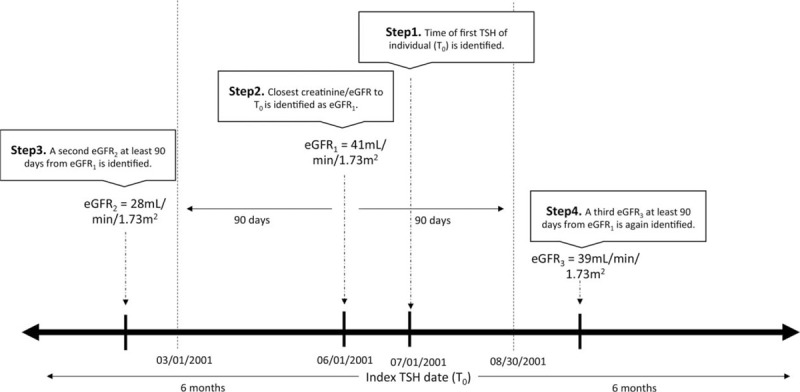
Example of identifying corresponding creatinine and eGFRs to index TSH date (T0). Starting from T0, July 1, 2001 in this individual's example, a query for the corresponding (most proximate) creatinine/eGFR within 6 months is performed, yielding eGFR1. A query for a second eGFR that is at least 90 days apart (outside the dotted lines) from eGFR1 but still within the 6 months time frame of T0 is performed, yielding eGFR2. Given that eGFR2 is of a different CKD class when compared with eGFR1, a query for a third (subsequent) eGFR that satisfies the above criteria is performed, yielding eGFR3. The average of the eGFR1 and eGFR3, in this case, 40 mL/min/1.73 m2, is taken and entered as the corresponding eGFR for this individual. CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, TSH = thyroid-stimulating hormone.
2.3. Study covariates
Information on demographics (including age, sex, and race/ethnicity), comorbidities (diabetes mellitus, hypertension, end stage renal disease on renal replacement therapy, polycystic kidney disease, glomerulonephritis, rheumatologic or autoimmune diseases, or renal transplant), laboratory results (thyroid-stimulating hormone [TSH], creatinine, estimated glomerular filtration rate [eGFR], hemoglobin A1c), and medication usage (anti-thyroid medications, thyroid hormone replacement medications, anti-hyperglycemic medications) were extracted. eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.[22] All disease conditions were identified using International Classification of Diseases (ICD) diagnosis codes unless otherwise specified. Diabetes was defined as having at least 1 hemoglobin A1c result ≥6.5% or at least 1 dispensation of anti-hyperglycemic medication (including any insulin and/or oral anti-hyperglycemic agents) within 6 months of the index TSH date. Hypertension was defined as at least 2 ICD codes regardless of encounter setting within 6 months of the index TSH date.[23] All medications were identified using Generic Product Identifier (GPI) codes. Information on pharmacy fills was retrieved from the KPSC pharmacy analytic database.
2.4. Exposure ascertainment
Thyroid status was categorized based on TSH values and thyroid hormone replacement and anti-thyroid medication use. TSH was measured using chemiluminescent microparticle immunoassay by Abbot Architect assays. The reference range of 0.35 to 4.00 mIU/L for normal was set based on the manufacturer, Abbot Diagnostic studies in conjunction with Southern California Permanente Medical Group Endocrinology Committee Review.[15,24] We adopted the upper and lower limits of this range as our cut-off. If there were multiple TSH measurements during the study window, the first TSH value was used and was considered the index TSH date.
Hyperthyroidism was defined as use of anti-thyroid medication (methimazole and/or propylthiouracil) and/or TSH level below the lower limit of normal (TSH <0.35 mIU/L). These patients were excluded in the study as noted above. The study population was categorized into hypothyroid and euthyroid individuals based on thyroid function. Hypothyroidism was defined as an elevated level of TSH (>4.00 mIU/L) and/or receipt of thyroid hormone replacement (levothyroxine sodium, thyroid, thyroid strong, bovine thyroid, pork thyroid, liothyronine sodium, and liotrix). Hypothyroidism was further classified into those with a hypothyroid status and those with an attenuated-hypothyroid status. Hypothyroid status was defined as a TSH >4 mIU/L regardless of thyroid hormone replacement while attenuated-hypothyroid status was defined as a TSH <4 mIUl/L and on thyroid hormone replacement. Individuals were considered to be on a medication if it was dispensed within 6 months of the index TSH date.
2.5. Main measures
Our primary measure was CKD defined as an eGFR <45 mL/min/1.73 m2. Our secondary measure was CKD defined as eGFR <60 mL/min/1.73 m2. Using the index TSH date, a query for corresponding creatinine values within 6 months of the index TSH date was performed. The most proximate creatinine to the index TSH date was used. Once this creatinine value was identified and its respective eGFR was calculated, a second creatinine and eGFR value at least 90 days apart from the initial eGFR but within the same 6-month window (to index TSH date) was obtained. If the second eGFR was within the same CKD stage as defined by Kidney Disease Improving Global Outcomes (KDIGO), the average of the 2 eGFRs was taken.[25] If the second eGFR identified was of a distinct CKD stage, a query for a third (subsequent) creatinine and its respective eGFR within the same timeframe criteria was performed until at least 2 eGFR values fell in the same CKD stage. An average of the 2 eGFRs (from the 2 queries matching in CKD stage) was used to report the eGFR for each individual (Fig. 1).
2.6. Statistical analysis
Prevalence of CKD by thyroid status were determined and compared along with demographics, comorbidities, and laboratory values. Chi-squared test was used for comparison of categorical variables, and the 2 sample Wilcoxon rank-sum test was used for continuous variables. Age and eGFR were reported as means and standard deviations. Sex, race/ethnicity, presence of diabetes, hypertension, and thyroid medication use were reported as an absolute number and percentage. The number and percentage of individuals within each thyroid status were classified into different CKD stages as defined by KDIGO.[25]
Multivariable logistic regression analysis was performed to estimate CKD odds ratio (OR) by thyroid status using euthyroid individuals as reference. Sequential adjustment based on baseline demographics including age, sex, and race/ethnicity, followed by comorbidities of diabetes and hypertension were performed. All statistical analyses were conducted using SAS statistical software (version 9.2, SAS Institute Cary, NC).
3. Results
3.1. Study population
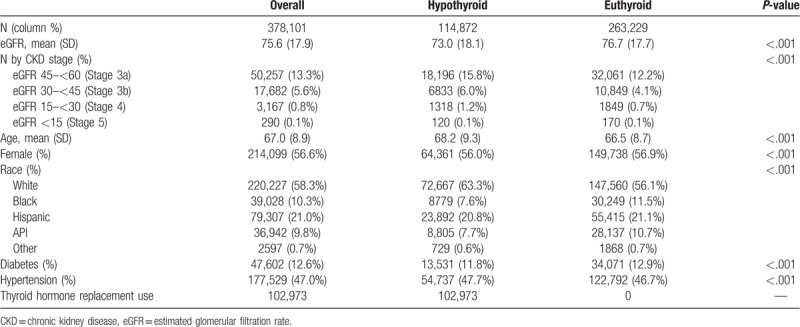
A total of 441,485 individuals who had outpatient serum TSH and creatinine measurements were identified. After 63,384 individuals were excluded based on exclusion criteria, a total of 378,101 individuals were included in our study population. (Fig. 2) Among this cohort, 114,872 (30.4%) individuals had hypothyroidism with 31,242 (27.2%) who had hypothyroid status and 83,630 (72.8%) who had attenuated-hypothyroid status (Fig. 1). The mean (±SD) age of the cohort was 67 (±9) years and 57% of patients were women. Whites, blacks, Hispanics, and Asian/Pacific Islanders comprised 58%, 10%, 21%, and 10% of the cohort, respectively. The mean (±SD) eGFR was 76 (±18) mL/min/1.73 m2. The cohort was comprised of 13% with diabetes and 47.0% with hypertension (Table 1).
Figure 2.
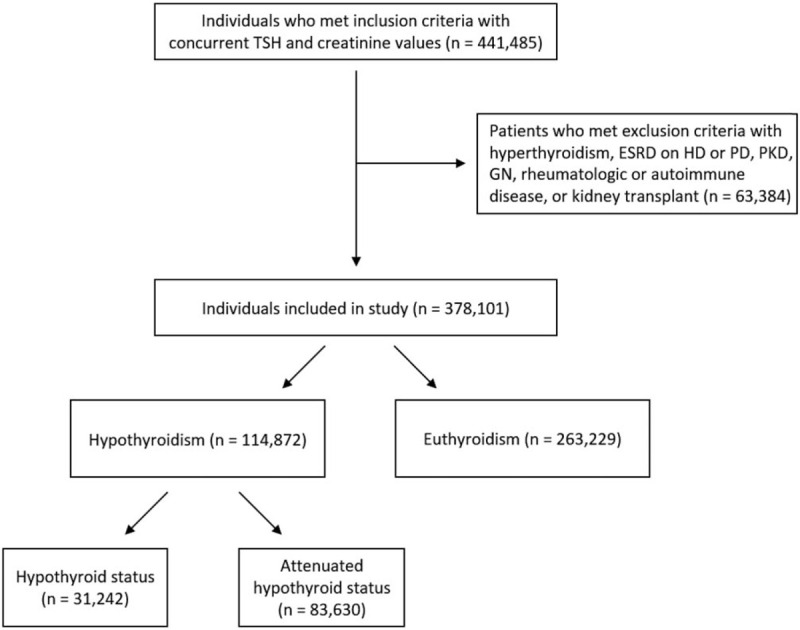
Study population. A total of 441,485 individuals who had outpatient serum TSH and creatinine measurements were identified. After 63,384 patients were excluded based on exclusion criteria, 378,101 individuals were included in our study population. Among this cohort, 114,872 (30.4%) individuals had hypothyroidism, among whom 31,242 (27.2%) had hypothyroid status and 83,630 (72.8%) had attenuated-hypothyroid status. TSH = thyroid-stimulating hormone.
Table 1.
Study cohort characteristics by thyroid status.
3.2. Prevalence of CKD by thyroid status
Among individuals with hypothyroidism, a total of 8271 (7.2%) had CKD (defined as an eGFR <45 mL/min/1.73 m2) compared with 12,868 (4.9%) with euthyroidism (P < .001) (Table 1). Within the hypothyroidism population, CKD was present in 2937 (9.4%) individuals with hypothyroid status compared with 5334 (6.4%) with attenuated hypothyroid status.
Using eGFR <60 mL/min/1.73 m2 to define CKD, a total 26,467 (23.0%) individuals with hypothyroidism had CKD compared with 44,929 (17.1%) with euthyroidism (P < .001). (Table 1). Within the hypothyroidism population, 8574 (27.4%) hypothyroid status individuals had CKD compared with 17,893 (21.4%) with attenuated hypothyroid status.
3.3. Regressions analysis for thyroid status and CKD
The crude OR (95% CI) for CKD (defined as eGFR <45 mL/min/1.73 m2) was 1.51 (1.47–1.55) for individuals with hypothyroidism compared with those with euthyroidism. Compared with euthyroid individuals, the crude ORs (95% CI) for CKD were 2.02 (1.94–2.11) and 1.33 (1.28–1.37) for hypothyroid and attenuated-hypothyroid status, respectively. The fully adjusted CKD OR (95% CI) was 1.25 (1.21–1.29) for individuals with hypothyroidism compared with euthyroidism. The adjusted CKD ORs (95% CI) were 1.59 (1.52–1.66) and 1.12 (1.08–1.16) for individuals with hypothyroid and attenuated-hypothyroid status, respectively (Table 2).
Table 2.
Odds ratio of CKD across each thyroid status.
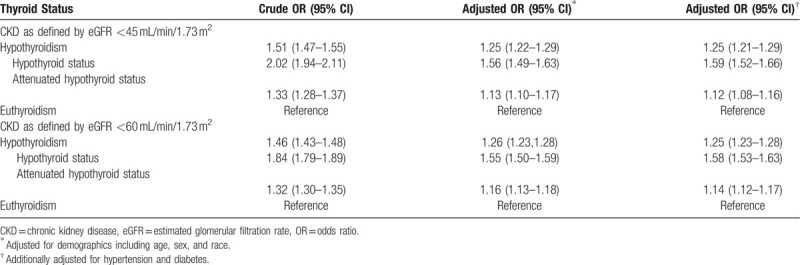
Using eGFR <60 mL/min/1.73 m2 to define CKD, the adjusted OR (95% CI) for CKD was 1.25 (1.23–1.28) among individuals with hypothyroidism. Among hypothyroid and attenuated-hypothyroid status individuals, the adjusted ORs (95% CI) for CKD were 1.58 (1.53–1.63) and 1.14 (1.12–1.17) respectively (Table 2).
4. Discussion
In this study of 378,101 individuals age 55 years and older with serum TSH and serum creatinine measurements, we observed a higher prevalence of CKD among individuals with hypothyroidism compared with those with euthyroidism. Individuals with hypothyroidism were 25% more likely to have CKD compared with those who were euthyroid. Upon examining more granular categories of hypothyroidism, we also found that there was a stronger association with CKD by thyroid status. Specifically, individuals with a hypothyroid status and an attenuated-hypothyroid status had 60% and 10% more likelihood for CKD compared with those with euthyroidism. Our findings from a real-world clinical setting of a large diverse population in the United States suggest a potential dose dependent relationship between thyroid status and CKD.
There has been increasing recognition of an interplay between thyroid status and kidney function.[4–12] A study of National Health and Nutrition Examination Survey (NHANES) III participants found that those with incrementally lower GFR had an increasingly higher prevalence of hypothyroidism.[6] These findings were corroborated in a study of the national Veterans Affairs (VA) population demonstrating an inverse relationship between lower eGFR and higher risk of hypothyroidism.[7] A prior study from Norway observed that lower thyroid function in clinically-normal ranges was associated with reduced eGFR.[8] Subsequent studies have shown that hypothyroidism even in the mild “subclinical” range is associated with decreased kidney function in other non-US populations.[11,12] However, interpretation of these data are limited by their non-consideration of a broader spectrum of thyroid function; restricted generalizability based on the size, race/ethnicity, or comorbidity status (i.e., diabetics only) of these study populations; and the sparse examination of potential confounders of thyroid status and kidney function.[8–12]
Hypothyroidism has been shown to cause changes in kidney structure and function in both development and adulthood. For example, animal models have shown that hypothyroidism leads to decreased kidney-to-body weight ratio, truncated tubular mass, changes in glomerular structure, decreased single nephron GFR, low renal plasma flow, and lower glomerular transcapillary hydrostatic pressure.[26–30] Case series have further shown hypothyroid patients have reduced renal plasma flow and GFR measured by creatinine-based estimating equations and gold-standard isotopic scans.[31–33] It has also been theorized that hypothyroidism leads to kidney dysfunction due to impaired systolic and diastolic function leading to reduced cardiac output, decreased vasodilator synthesis and activity leading to changes in intra-renal hemodynamics, altered RAAS, and increased tubulo-glomerular feedback.[13,34–38]
Diagnosis of hypothyroidism is simple, and its treatment is cost-effective and safe.[15] Yet, there are inconsistent recommendations with respect to the screening for hypothyroidism. At this time, the United States Preventive Service Task Force (USPSTF) does not recommend screening of hypothyroidism among asymptomatic non-pregnant adults.[16,17] The American Academy of Family Physicians also support the USPSTF recommendations.[18] In contrast, the American Thyroid Association (ATA) and the American Association of Clinical Endocrinologists (AACE) recommends that screening for hypothyroidism should be considered in patients age over 60, and that “aggressive case finding” should be considered in those at increased risk for hypothyroidism.[15] Furthermore, the American College of Cardiology and American Heart Association recommends thyroid function screening tests in patients with newly diagnosed heart failure, which may pertain to a large proportion of CKD patients.[39,40] Our study adds to the growing literature of the relationship between thyroid status and kidney function and may support screening of certain populations such as those with CKD. Given our findings, the attenuation of hypothyroid status or treatment of hypothyroidism may potentially impact renal function and CKD risk.
Our study has several potential limitations that may confound the interpretation of our findings. First, the cross-sectional design limits our ability to draw conclusions regarding the directionality between hypothyroidism and CKD. Our findings may be affected by selection bias in which individuals with a higher pre-test probability of thyroid and kidney dysfunction were more likely to undergo laboratory testing by their providers. Second, we used eGFR as our sole criteria for ascertaining CKD due to the lack of reliable information on other kidney disease markers (i.e., albuminuria, proteinuria). Third, our study was limited to those ages 55 and older, and thus we cannot generalize our findings to younger patients. Finally, thyroid status was designated based on a single TSH value without a free T4 value and a further confirmation of elevated TSH. TSH may be prone to fluctuations and/or individuals may have had transient hypothyroid states which may not be clinically relevant. Confirmation of hypothyroidism with additional thyroid function testing would be important to avoid potential overtreatment.
Despite these limitations, our study is one of the largest diverse populations within a real-world clinical environment examining the association between thyroid disease and CKD. Although our study population is of older age, CKD is disproportionately greater among older individuals, and the relevance of our findings likely remain largely significant and may even support the ATA/AACE recommendations for aggressive case finding among those age over 60 years of age.[15] In addition, while unable to take into account markers of kidney damage (i.e., albuminuria, proteinuria), we utilized a rigorous approach in ascertaining cases of CKD which only examined outpatient measurements and required at least 2 eGFR values separated by at least 90 days. Most importantly, our granular distinction of those with hypothyroid versus attenuated-hypothyroid status among those with hypothyroidism allowed us to identify a dose dependent relationship between thyroid status and CKD.
In conclusion, we observed that CKD was associated with hypothyroidism among individuals from a large diverse real-world population. Our findings also suggested a dose dependent relationship as those with hypothyroid status more than treated/attenuated hypothyroid patients had a stronger association with CKD. Future and more definitive studies are needed to examine whether correction of confirmed hypothyroidism with thyroid hormone replacement may ultimately improve kidney outcomes.
Acknowledgment
The authors would like to thank Ji Yeon Kim MD, Aida R. Legaspi, Chiemi Tabata, and Darryl Erik Palmer-Toy, MD, PhD from Southern California Permanente Medical Group Regional Reference Laboratories for their assistance and support on this project.
Author contributions
Acquisition, analysis or interpretation of data: Bonnie H. Li, Cheng-Wei Huang, Connie M. Rhee, John J. Sim.
Administrative, technical, or material support: Steven J. Jacobsen.
Critical revision of the manuscript for important intellectual content: Steven J. Jacobsen, Kristi Reynolds, Bonnie H. Li.
Data analysis: Bonnie H. Li.
Drafting of the manuscript: Cheng-Wei Huang, John J. Sim, Connie M. Rhee.
Statistical analysis: Bonnie H. Li.
Study concept and design: Cheng-Wei Huang, John J. Sim, Connie M. Rhee.
Study supervision: John J. Sim, Connie M. Rhee.
John J. Sim orcid: 0000-0001-9456-8243.
John J Sim orcid: 0000-0001-9456-8243.
Footnotes
Abbreviations: CKD = chronic kidney disease, eGFR = estimated glomerular filtration rate, EHR = electronic health records, KPSC = Kaiser Permanente Southern California, OR = odds ratio, RAAS = renin angiotensin aldosterone system, TSH = thyroid-stimulating hormone.
This study was supported by the Clinician Investigator Award (JJS) and The Regional Research Committee of Kaiser Permanente Southern California
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Huang CW, Li BH, Reynolds K, Jacobsen SJ, Rhee CM, Sim JJ. Association between hypothyroidism and chronic kidney disease observed among an adult population 55 years and older. Medicine 2020;99:17(e19569).
References
- [1]. Department of Health U, Services H, for Disease Control C. National Chronic Kidney Disease Fact Sheet, 2017 CKD Is Common Among Adults in the United States Fast Stats; 2017. Available at: https://www.cdc.gov/kidneydisease/pdf/kidney_factsheet.pdf. Accessed December 3, 2018. [Google Scholar]
- [2].USRDS. 2016 USRDS Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2017;67:S291–334. [Google Scholar]
- [3].Luyckx VA, Tonelli M, Stanifer JW. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ 2018;96:414–22D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chonchol M, Lippi G, Salvagno G, et al. Prevalence of subclinical hypothyroidism in patients with chronic kidney disease. Clin J Am Soc Nephrol 2008;3:1296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chandra A. Prevalence of hypothyroidism in patients with chronic kidney disease: a cross-sectional study from North India. Kidney Res Clin Pract 2016;35:165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lo JC, Chertow GM, Go AS, et al. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int 2005;67:1047–52. [DOI] [PubMed] [Google Scholar]
- [7].Rhee CM, Kalantar-Zadeh K, Streja E, et al. The relationship between thyroid function and estimated glomerular filtration rate in patients with chronic kidney disease. Nephrol Dial Transplant 2015;30:282–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Åsvold BO, Bjøro T, Vatten LJ. Association of thyroid function with estimated glomerular filtration rate in a population-based study: the HUNT study. Eur J Endocrinol 2011;164:101–5. [DOI] [PubMed] [Google Scholar]
- [9].Schultheiss UT, Daya N, Grams ME, et al. Thyroid function, reduced kidney function and incident chronic kidney disease in a community-based population: the Atherosclerosis Risk in Communities study. Nephrol Dial Transplant 2017;32:1874–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gopinath B, Harris DC, Wall JR, et al. Relationship between thyroid dysfunction and chronic kidney disease in community-dwelling older adults. Maturitas 2013;75:159–64. [DOI] [PubMed] [Google Scholar]
- [11].Zhou J-B, Li H-B, Zhu X-R, et al. Subclinical hypothyroidism and the risk of chronic kidney disease in T2D subjects. Medicine (Baltimore) 2017;96:e6519. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [12].Chang Y-C, Chang CH, Yeh Y-C, et al. Subclinical and overt hypothyroidism is associated with reduced glomerular filtration rate and proteinuria: a large cross-sectional population study. Sci Rep 2018;8:2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rhee CM. The interaction between thyroid and kidney disease: an overview of the evidence. Curr Opin Endocrinol Diabetes Obes 2016;23:407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489–99. [DOI] [PubMed] [Google Scholar]
- [15].Garber J, Cobin R, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 2012;18:988–1028. [DOI] [PubMed] [Google Scholar]
- [16].LeFevre ML. Screening for thyroid dysfunction: U.S. preventive services task force recommendation statement. Ann Intern Med 2015;162:641–50. [DOI] [PubMed] [Google Scholar]
- [17].Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med 2015;162:35–45. [DOI] [PubMed] [Google Scholar]
- [18]. American Academy of Family Physicians. Summary of Recommendations for Clinical Preventive Services Introduction to AAFP Summary of Recommendations For Clinical Preventive Services; 2017. Available at: https://www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/cps-recommendations.pdf. Accessed June 16, 2019. [Google Scholar]
- [19].Adams AL, Li BH, Bhandari S, et al. Chronic hyponatremia and association with osteoporosis among a large racially/ethnically diverse population. Osteoporos Int 2019;30:853–61. [DOI] [PubMed] [Google Scholar]
- [20].Derose SF, Contreras R, Coleman KJ, et al. Race and ethnicity data quality and imputation using U.S. Census Data in an Integrated Health System. Med Care Res Rev 2013;70:330–45. [DOI] [PubMed] [Google Scholar]
- [21].Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau Data. Perm J 2012;16:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sim JJ, Zhou H, Bhandari S, et al. Low systolic blood pressure from treatment and association with serious falls/syncope. Am J Prev Med 2018;55:488–96. [DOI] [PubMed] [Google Scholar]
- [24]. SCPMG Regional Reference Core Laboratories. TSH: Test Information. SCPMG Lab Net; 2018. Available at: http://kpnet.kp.org:81/california/scpmg/labnet/testmenu/testmenu.jsp?TID=2755. Accessed June 16, 2019. [Google Scholar]
- [25].Andrassy KM. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int 2013;84:622–3. [DOI] [PubMed] [Google Scholar]
- [26].Rodríguez-Gómez I, Banegas I, Wangensteen R, et al. Influence of thyroid state on cardiac and renal capillary density and glomerular morphology in rats. J Endocrinol 2013;216:43–51. [DOI] [PubMed] [Google Scholar]
- [27].Slotkin TA, Seidler FJ, Kavlock RJ, et al. Thyroid hormone differentially regulates cellular development in neonatal rat heart and kidney. Teratology 1992;45:303–12. [DOI] [PubMed] [Google Scholar]
- [28].Canavan JP, Holt J, Easton J, et al. Thyroid-induced changes in the growth of the liver, kidney, and diaphragm of neonatal rats. J Cell Physiol 1994;161:49–54. [DOI] [PubMed] [Google Scholar]
- [29].Bentley AG, Madsen KM, Davis RG, et al. Response of the medullary thick ascending limb to hypothyroidism in the rat. Am J Pathol 1985;120:215–21. [PMC free article] [PubMed] [Google Scholar]
- [30].Bradley SE, Coelho JB, Sealey JE, et al. Changes in glomerulotubular dimensions, single nephron glomerular filtration rates and the renin-angiotensin system in hypothyroid rats. Life Sci 1982;30:633–9. [DOI] [PubMed] [Google Scholar]
- [31].Kreisman SH, Hennessey JV. Consistent reversible elevations of serum creatinine levels in severe hypothyroidism. Arch Intern Med 1999;159:79–82. [DOI] [PubMed] [Google Scholar]
- [32].Villabona C, Sahun M, Roca M, et al. Blood volumes and renal function in overt and subclinical primary hypothyroidism. Am J Med Sci 1999;318:277–80. [DOI] [PubMed] [Google Scholar]
- [33].Karanikas G, Schütz M, Szabo M, et al. Isotopic renal function studies in severe hypothyroidism and after thyroid hormone replacement therapy. Am J Nephrol 2004;24:41–5. [DOI] [PubMed] [Google Scholar]
- [34].Mariani LH, Berns JS. The renal manifestations of thyroid disease. J Am Soc Nephrol 2012;23:22–6. [DOI] [PubMed] [Google Scholar]
- [35].Falk SA, Buric V, Hammond WS, et al. Serial glomerular and tubular dynamics in thyroidectomized rats with remnant kidneys. Am J Kidney Dis 1991;17:218–27. [DOI] [PubMed] [Google Scholar]
- [36].Bradley SE, Stéphan F, Coelho JB, et al. The thyroid and the kidney. Kidney Int 1974;6:346–65. [DOI] [PubMed] [Google Scholar]
- [37].Basu G, Mohapatra A. Interactions between thyroid disorders and kidney disease. Indian J Endocrinol Metab 2012;16:204–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rhee CM, Brent GA, Kovesdy CP, et al. Thyroid functional disease: an under-recognized cardiovascular risk factor in kidney disease patients. Nephrol Dial Transplant 2015;30:724–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fuster V, Rydén LE, Asinger RW, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) Developed in Collaboration With the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol 2001;38:1231–65. [DOI] [PubMed] [Google Scholar]
- [40].Sim JJ, Zhou H, Shi J, et al. Disparities in early mortality among chronic kidney disease patients who transition to peritoneal dialysis and hemodialysis with and without catheters. Int Urol Nephrol 2018;50:963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


