Scheme 1.
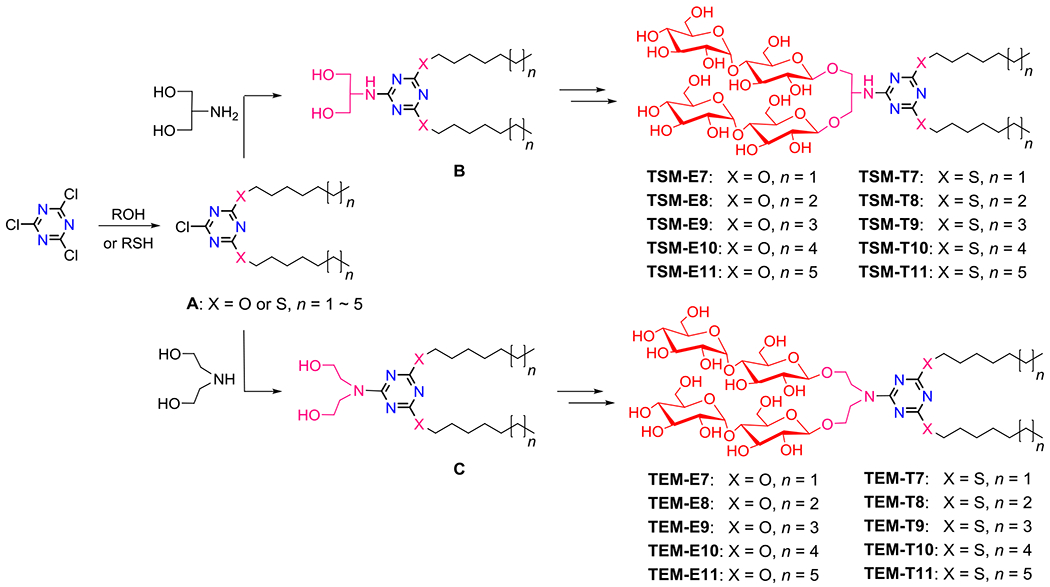
Synthetic schemes for generation of TSMs and TEMs. Two alkyl chains were introduced into the 1,3,5-triazine unit by reacting two equivalents of an alkanol (ROH; TSM-Es/TEM-Es) or an alkanethiol (TSM-Ts/TEM-Ts) with cyanuric chloride. The dialkylated products with the ether or thioether linkage (A) were further modified by an amine-functionalized linker (serinol/diethanolamine (DEA) for the TSMs/TEMs), giving a dialkylated 1,3,5-triazine-cored diol derivative (B/C). The resulting diol derivatives (B and C) were additionally decorated with two maltose groups via β-stereoselective glycosylation and global deprotection, affording the final amphiphilic compounds (TSMs or TEMs). Variations in the amine-functionalized linker (serinol/DEA), alkyl linkage (ether/thioether) and the alkyl chain lengths (from C7 to C11) are indicated in the detergent designation.
