Figure 4.
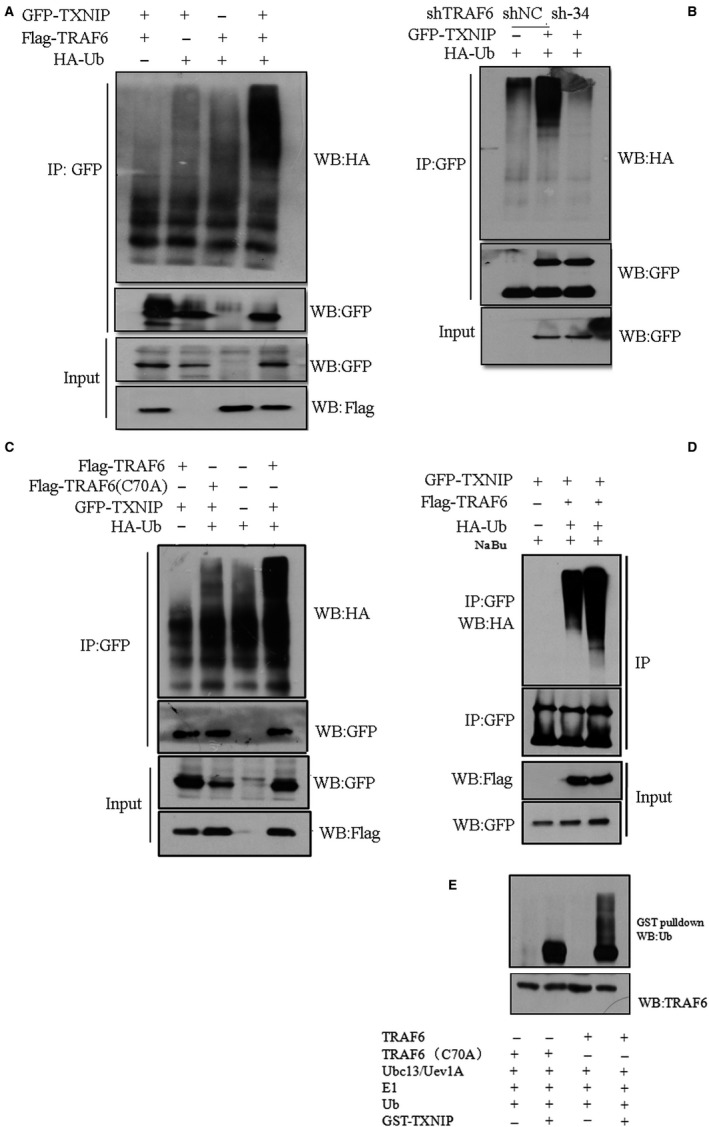
TRAF6 polyubiquitylated TXNIP. A, Expression construct of GFP‐TXNIP was co‐transfected with Flag‐TRAF6 or corresponding empty vector and/or HA‐Ub/ empty vector into HEK293T cells. After 36 h transfection, cells were lysated with 1 × RIPA buffer containing complete inhibitors and used for IP assay with anti‐GFP antibody. During the washing step, we added 6 mol/L urea into the washing buffer at the second wash step. Thereafter, immunoblotting assay was performed with anti‐HA (IP)/anti‐Flag (Lysates)/anti‐GFP(IP/Lysates) antibody. B, A549 cells stably expressing TRAF6 shRNA (sh‐34) or scramble RNA (shNC) were co‐transfected with GFP‐TXNIP or its corresponding empty vector together with vector expressing HA‐Ub. IP was performed with anti‐GFP antibody post‐48 h transfection. The eluted IP protein was subjected to western blot with Anti‐HA antibody or anti‐GFP antibody. C, GFP‐TXNIP construct was co‐transfected with Flag‐TRAF6 (wild type) or Flag‐TRAF6 (C70A mutant) constructs together HA‐Ub construct into HEK293T cells. The IP process is the same as A, or B. anti‐HA antibody was used for the detection of polyubiqutylation of TXNIP. D, the plasmids transfected into HEK293T cells was the same as in A. After transfection, cells were treated with 2 mmol/L NaBu for 24 h, and IP was performed as A using GFP antibody. Anti‐HA antibody was used for the immunoblotting detection. E, Bacterially expressed and purified GST‐TXNIP proteins were incubated with Flag‐TRAF6 or Flag‐TRAF6/C70A mutant in the presence of E1, E2 (Ubc13/Uev1A), and ubiquitin (Ub). Following the ubiquitination reaction, the TXNIP‐ubiquitin conjugates were detected by GST pull‐down and immunoblotted with anti‐Ub, anti‐GST and anti‐TRAF6, respectively
