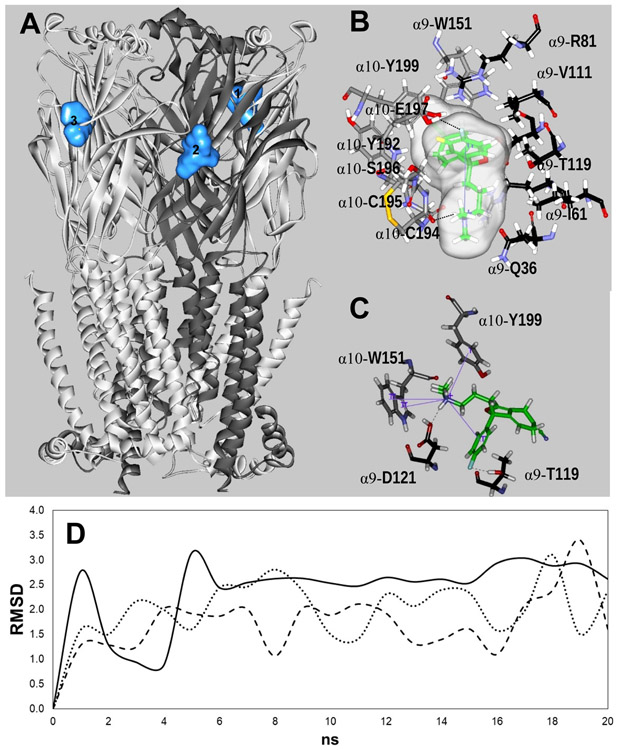
Figure 6.
Docking sites for S-(+)-citalopram (escitalopram) at the h(α9)2(α10)3 AChR model. (A) Escitalopram interacted with three possible orthosteric sites located at the interface between the (+)α10 (principal component) and (−)α9 [or another (−)α10] subunit (complementary component) (light blue surface models). α10 (white) and α9 (dark grey) subunits are represented as solid ribbons. (B) In site 1, escitalopram (as sticks and its transparent surface model, colored by atoms with carbons in green) formed a strong H-bond with two oxygens, one on the α10-C194 main chain and another on the α10-E197 side chain (dotted black line). Interestingly, an intramolecular cation-π interaction is formed in escitalopram, between N+ and its fluorophenyl ring (solid blue line). (C) In site 2, escitalopram (as sticks colored by atoms with carbons in green) formed a network of H-bond and cation-π interactions. Other details are included in Figure 5. The complete list of residues interacting at each site is summarized in Table 5. (D) Molecular dynamics simulations (20 ns) of escitalopram interacting with sites 1 (-----), 2 (—), and 3 (…··), respectively, at the h(α9)2(α10)3 model.