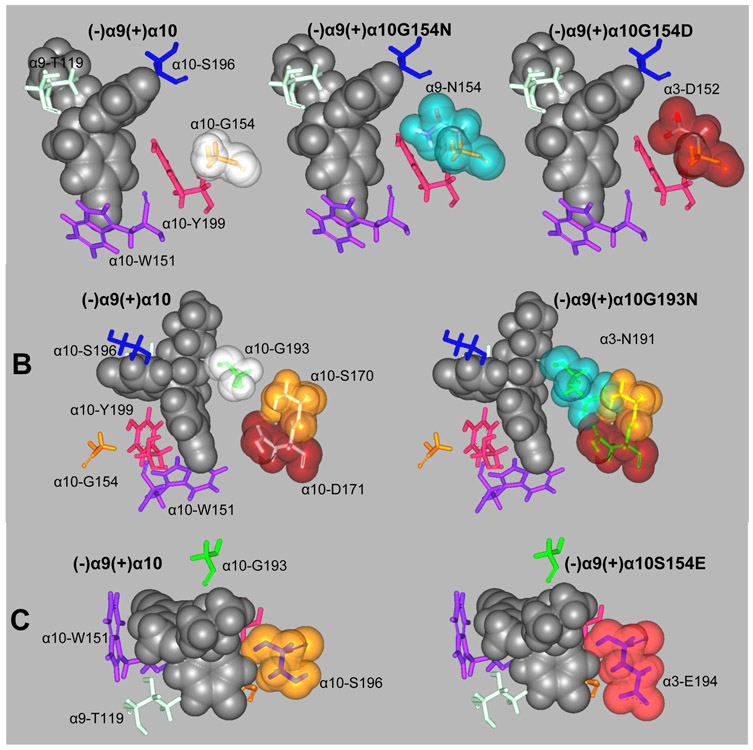
Figure 7.
In silico mutations of (+)α10 residues involved in escitalopram binding to orthosteric sites to their respective homologous residues at α9 and α3. (A) α10-G154 mutations to its α9 (α10G154N) and α3 (α10G154D) homologous residues. (B) α10-G193 mutation to its α3 homologous residue (α10G193N). (C) α10-S196 mutation to its α3 homologous residue (α10S196E). Escitalopram is represented by its van der Waal solid surface in black. Mutated residues are represented as sticks surrounded by their van der Waal transparent surfaces. Other important binding site residues are represented as sticks.