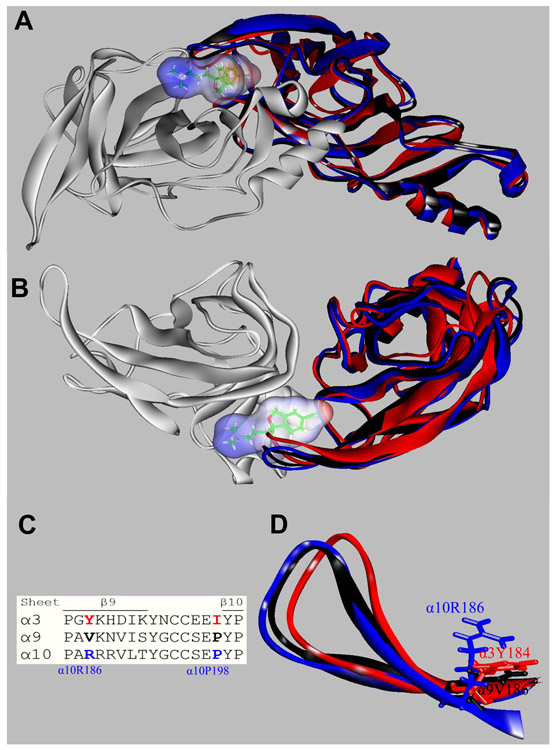
Figure 8.
Orthosteric binding sites at the superposed (+)α3 (red), (+)α9 (black), (+)α10 (blue), and (−)α9 (white) subunits. Escitalopram is shown as sticks surrounded by its molecular surface. The β9-β10 loop at the α3 and α9 subunits are closer to the receptor center than that at α10 (A: Extracellular view; B: Cytoplasmic view), and consequently there is no room for escitalopram to fit in the agonist binding site in α3 and α9. The α3- and α9-β9-β10 loops overlap the ligand when is docked as in the (α9)2(α10)3 receptor. (C) Amino acid sequence comparison between α3, α9, and α10 subunits at the level of the β9-β10 loop. Blue: amino acids identified as the cause of the different β9-β10 loop conformations (see text). (D) A detailed side chain view at α10R186, α9V186, and α3Y184 positions, showing the differences of side chains occupied volume that would force the α10-β9-β10 loop to set apart from the receptor.