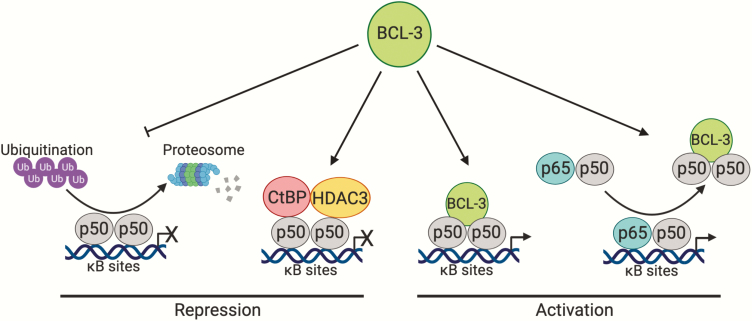
Figure 2.
BCL-3 regulation of the atypical NF-κB signalling pathway. The atypical pathway is defined as the activation of the NF-κB p50 and p52 homodimers. BCL-3 acts as a regulator of the atypical NF-κB pathway by binding to processed p50 and p52 homodimers to repress or activate a subset of NF-κB regulated genes. In terms of transcriptional regulation, BCL-3 stabilizes repressive p50 homodimers bound to κB sites by inhibiting ubiquitination and subsequent proteolytic degradation of p50 homodimers. Additionally, BCL-3 can also recruit co-repressors CtBP and HDAC3 to repress transcription of NF-κB target genes. Conversely, BCL-3 directly activates transcription of NF-κB target genes by associating with p50 and p52 homodimers, providing these homodimers with two transactivation domains that they otherwise lack. BCL-3 can also remove repressive p50 homodimers situated at κB sites on DNA, allowing NF-κB heterodimers (p65/p50) associated with canonical signalling to activate transcription at these sites. Whilst BCL-3 is known to directly interact with p52 homodimers, the mechanism by which BCL-3 regulates p52 homodimer activation remains to be elucidated however it is speculated to follow a similar mechanism to p50 homodimers. Figure created with BioRender.com.