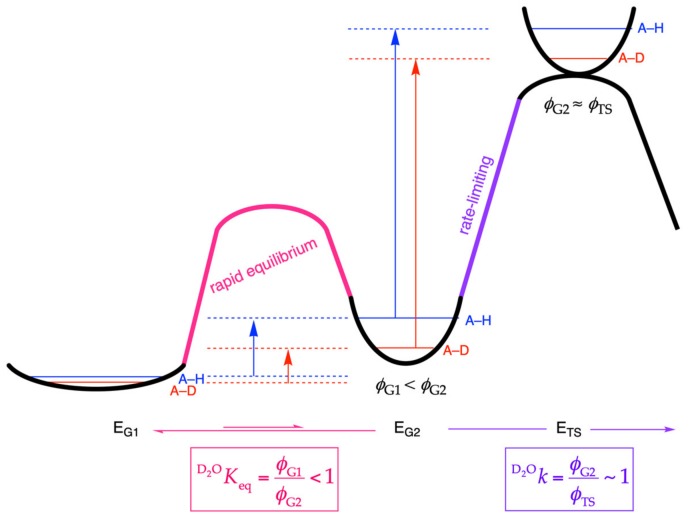
Figure 1.
Reaction coordinate diagram of a solvent-sensitive rapid equilibrium preceding a rate-limiting step that results in inverse solvent isotope effects. Horizontal lines represent zero-point energy levels, reflecting the mass of the hydron and the stiffness of the bond in which it is involved. When the stiffness of the bond increases, as shown in the rapid-equilibrium step (pink), the zero-point energy difference between the two isotopes increases relative to the reactant ground state (G1), leading to accumulation of deuterium in the intermediate ground state (G2); this is reflected in an increase in the fractionation factor (ϕ; Equation (1)) for this step and an inverse solvent equilibrium isotope effect (SEIE) (i.e., D2OKeq < 1). If the stiffness of the solvent-exchangeable hydron is not altered in the transition state (TS) of a subsequent rate-limiting step (purple), then no SKIE will be contributed for this step (i.e., D2Ok ~ 1). The observed inverse solvent kinetic isotope effect (SKIE) (D2Okobs) for the overall process will be inverse (Equation (2)). A = solvent-exchangeable group; E = enzyme.