Figure 4. Overview of sugar and lipid metabolic pathways affected by oxygen availability.
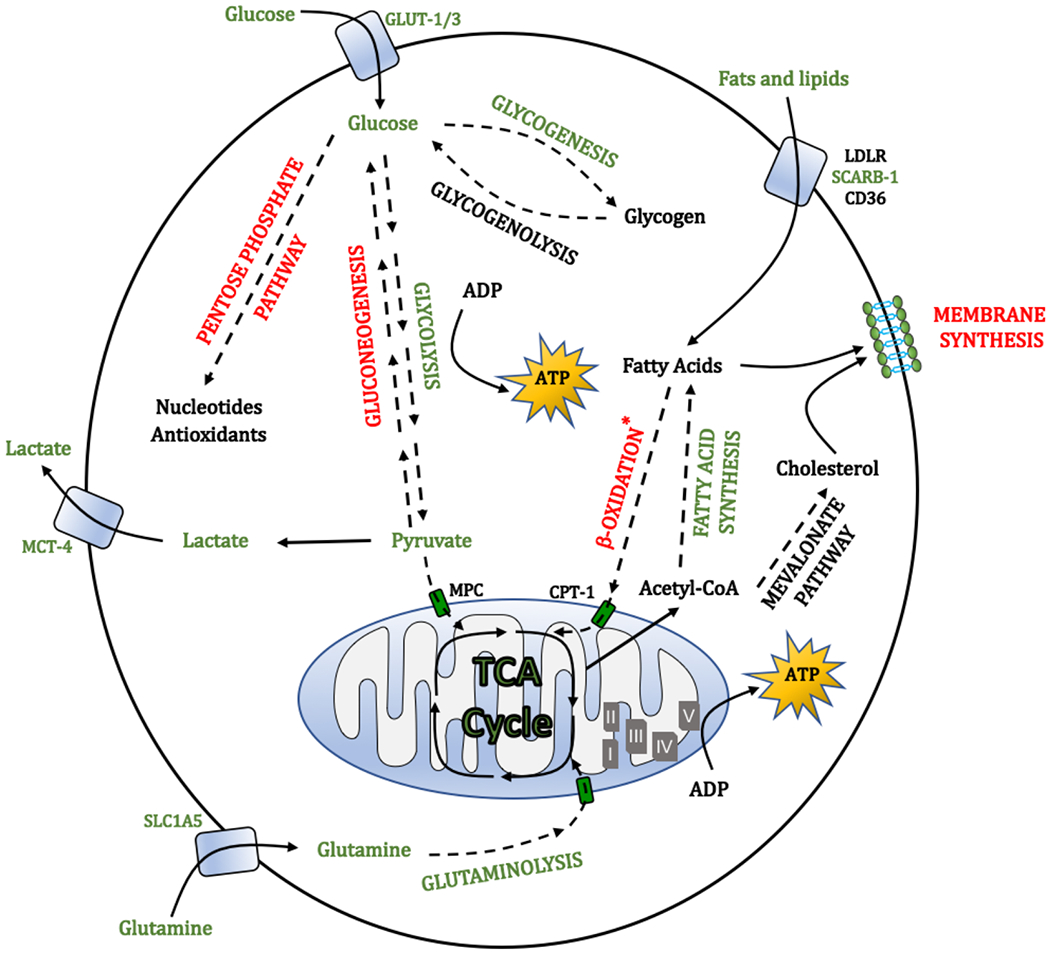
The figure highlights the upregulated (green) and downregulated (red) metabolic pathways under hypoxia. Hypoxic cells increase the uptake and utilization of glucose via glycolysis to obtain energy, while reducing their metabolism in mitochondria (Fig. 3). This leads to the generation of large amounts of lactate, which is secreted and can support metabolism of neighbouring cells. As a protective mechanism, glucose is also diverted towards the serine synthesis pathway to overcome the loss of cellular antioxidant capacity when pentose phosphate pathway activity is decreased. Hypoxia also promotes glycogenesis, which could provide a mechanism of energy storage to survive prolonged stress. Under hypoxic inhibition of the tricarboxylic acid (TCA) cycle, generation of anabolic metabolites, such as acetyl-CoA, which is key for the synthesis of fatty acids, is largely supported by the increased uptake and metabolism of glutamine. Concomitantly, catabolism of fatty acids via β-oxidation is suppressed, while the uptake of lipids from the exterior increased. Lipid desaturation, allowing generation of unsaturated fatty acids is inhibited in hypoxia (owing to the requirement for oxygen by stearoyl-CoA desaturase). To counteract potential lipotoxicity associated with the accumulation of saturated lipids and disruption of membrane structure and integrity, cells increase the uptake of unsaturated lipids from the environment and increase the formation of lipid droplets, which can act as buffers for saturated lipid species. ADP, adenosine diphosphate; ATP, adenosine triphosphate; CD36, cluster of differentiation 36; CPT-1, carnitine palmitoyltransferase 1; ETC, electron transport chain; GLUT-1, glucose transporter 1; LDLR, low density lipoprotein receptor; MCT4, monocarboxylate transporter 4; MPC, mitochondrial pyruvate carrier; SCARB-1, scavenger receptor B1; SLC1A5, solute carrier family 1 (neutral amino acid transporter) member 5.
