Fig. 2.
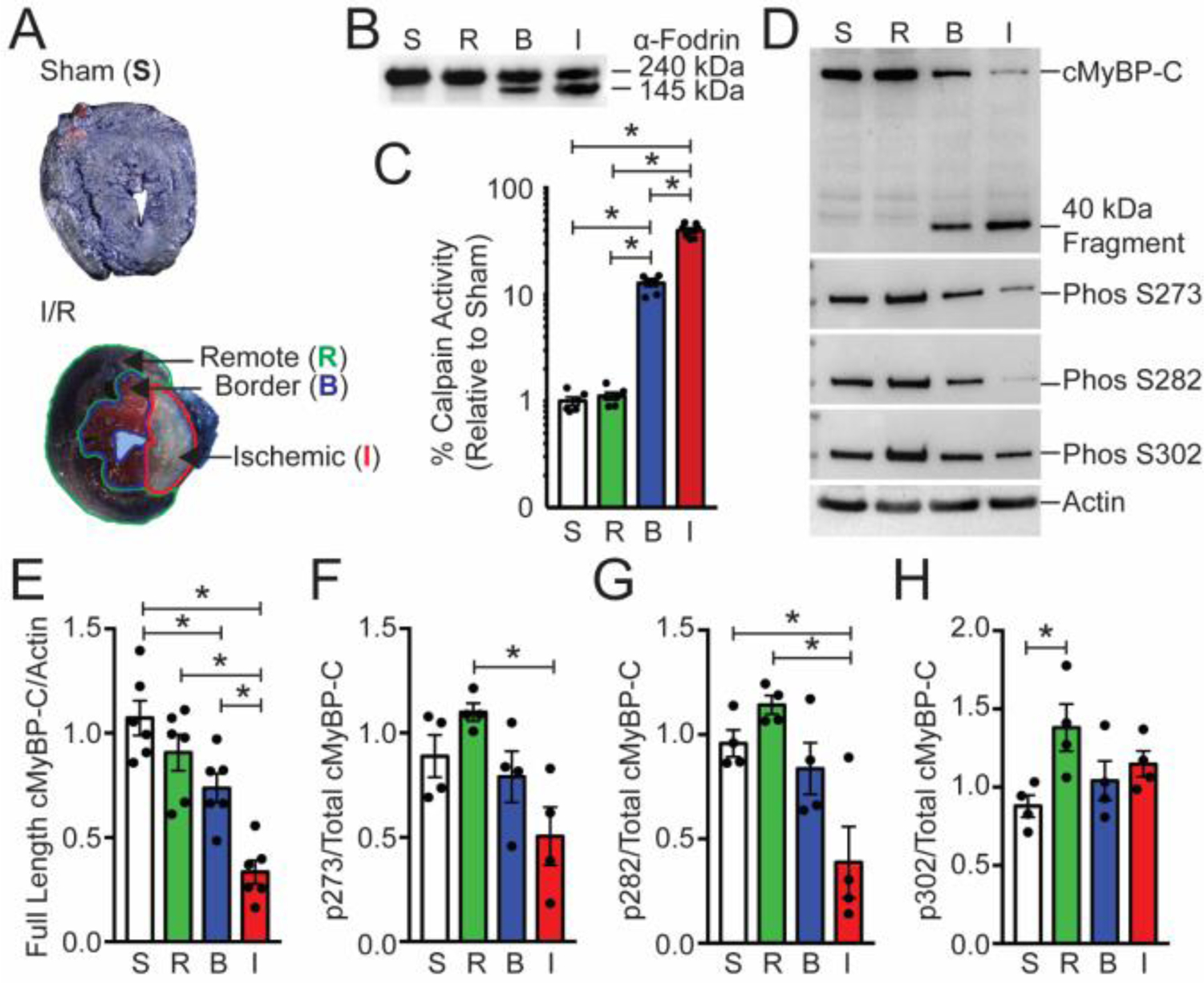
Cardiac MyBP-C is dephosphorylated and degraded in a mouse ischemia/reperfusion model. (A) TTC-staining of I/R-injured mouse hearts showed the remote regions stained blue [R], the border region stained pink [B], and the infarct [I] region in white. The sham heart [S] was stained with TTC and blue dye and shows uniform blue staining. (B) The calpain proteolysis target α-fodrin shows degradation in protein samples from the border and ischemic regions. (C) Calpain activity was determined from sham, remote, border, and infarcted regions (n = 6, S; 6, R; 7, B; 7, I). (D) The levels of cMyBP-C from these regions were determined using Western blot with the anti-cMyBP-C2–14 antibody, and the extent of cMyBP-C degradation was evaluated. The presence of the 40kDa cMyBP-C N’-terminal fragment was identified in the remote and infarct area. Sarcomeric actin was used as a loading control (lower panel). Western blot analysis of cMyBP-C was performed to determine the level of cMyBP-C phosphorylation using phospho-specific antibodies. (E) Quantification of total cMyBP-C and (F–H) phosphorylated cMyBP-C at residues S273, S282, and S302 (n = 6, total protein; 4, phospho-protein). All data are mean ± SEM (* P<0.05; one-way ANOVA with Holm-Sidak post-hoc test).
