Abstract
Patients with multiple sclerosis (MS) are at increased risk of infections and related worsening of neurological function. Influenza infection has been associated with increased risk of various neurological complications. We conducted a population-based registry study to investigate the risk of acute hospitalization of MS patients in relation to influenza infection or pandemic vaccination in Norway. The entire Norwegian population in the years 2008–2014 was defined as our study population (N = 5,219,296). Information on MS diagnosis, influenza infection and vaccination were provided by Norwegian national registries. The self-controlled case series method was used to estimate incidence rate ratios (IRRs) with 95% confidence intervals (95% CI) in defined risk periods. 6755 MS patients were identified during the study period. Average age at first registration of an MS diagnosis was 51.8 years among men and 49.9 years among females (66.9%). The IRR for emergency hospitalization among MS patients the first week after an influenza diagnosis was 3.4 (95% CI 2.4–4.8). The IRR was 5.6 (95% CI 2.7–11.3) after pandemic influenza, and 4.8 (95% CI 3.1–7.4) after seasonal influenza. Pandemic vaccination did not influence risk of hospitalization [IRR within the first week: 0.7 (95% CI 0.5–1.0)]. Among MS patients, influenza infection was associated with increased risk for acute hospitalization while no increased risk was observed after pandemic vaccination. Influenza vaccination could prevent worsening of MS-related symptoms as well as risk of hospitalization.
Keywords: Multiple sclerosis, Influenza, Pandemic, Pandemrix vaccination, Hospitalization, Norway
Introduction
Multiple sclerosis (MS) is a chronic neurological disease with unknown aetiology and unpredictable progress. Immunological mechanisms are thought to play an important role in the development of MS due to interaction between environmental and genetic risk factors [1]. MS patients are at increased risk of infections, as disease-modifying therapies may alter the normal function of the immune system, or due to underlying changes in immune response [2]. Although hospitalization rates among MS patients have declined over the past few decades, they remain generally higher than in the general population [3–5]. MS-related factors and infections are the main causes of hospitalizations among MS patients. Increased exacerbation rates in MS patients have been reported after influenza illness [6] similar to what has been observed after systemic or general viral infections [7–9]. Viral infections have been shown to be associated with clinical activity of MS [2, 8–10], hence infectious diseases such as influenza may induce relapses and cause acute worsening of neurologic function, while vaccination against influenza may prevent such adverse events [11, 12].
Previous studies have mainly focused on influenza vaccination and risk of MS relapse [12, 13]. In this large register-based study we provide a comprehensive assessment of hospitalization risk after specifically influenza infection. We aim to examine risk of acute hospitalization among MS patients up to 3 months following influenza infection and pandemic vaccination in a large population-based cohort consisting of 6755 MS patients using nationwide population-based Norwegian registries.
Methods
Study population
The study population consisted of all Norwegian residents during the period 2008–2014 (N = 5,219,296) as registered in the Norwegian National Registry. This registry provided information on date of birth, date of emigration, and death.
Data sources
We linked information from several national health registries and databases. All registries contain the personal identification number (PIN) which is unique for all citizens, enabling linkage of information at the individual level. Information from hospitals and specialist care were obtained from the Norwegian Patient Registry (NPR). The NPR is an administrative database linked to the reimbursement system with mandatory reporting [14]. Diagnoses are reported according to the International Classification of Disease, version 10 (ICD-10). The NPR provided information on hospitalizations with ICD-10 code G35 (“Multiple sclerosis”).
Information on influenza diagnoses in primary care was retrieved from the Norwegian Directorate of Health which reimburses consultations in emergency outpatient clinics and general practice. Influenza diagnoses in primary care were based on the International Classification of Primary Care, second edition (ICPC-2) code R80 (“Influenza”). The criteria for using the R80 code include specific symptoms (e.g. rapid onset, chills/fever and fatigue) and ongoing influenza epidemic or influenza in the community. These criteria have been described in detail previously [15, 16]. In addition to the clinical diagnoses of influenza, we obtained information on pandemic influenza infections from the Norwegian Surveillance System for Communicable Diseases to which laboratory-confirmed pandemic influenza (but not seasonal influenza) was reported [17].
Dates of pandemic vaccinations were obtained from the Norwegian Immunisation Registry [18]. The Pandemrix® vaccine was offered to the whole population. Notification of pandemic influenza vaccinations to the Norwegian Immunisation Registry was mandatory.
Information on medical prescriptions with MS specific medications dispensed from Norwegian pharmacies was provided by the Norwegian Prescription Database (NorPD) from 2004 onwards [19]. The Anatomical Therapeutic Chemical Classification System (ATC) codes for MS related medications were L03AX13 Copaxone® (glatiramer acetate), L04AA27 Gilenya® (fingolimod), L04AA31 Aubagio® (teriflunomide), N07XX09 Tecfidera® (dimethyl fumarate), L03AB07 Avonex®, Rebif® (interferon beta 1a), L03AB08 Betaferon®, Extavia® (interferon beta 1b), L03AB13 Plegridy® (peginterferon beta 1 a). Medication with ATC codes L04AA23 Tysabri® (natalizumab) and L04AA34 Lemtrada® (alemtuzumab) are administered in hospitals only, and were not included. The NorPD does not hold individual-level information on medication administered during hospitalizations.
Definition of MS patients and outcome
An MS patient was defined by having at least one MS diagnosis registered in specialist care, combined with at least one dispensed MS medication (54% of patients). If lacking information on medication use, it was required to have at least two registrations of an MS diagnosis (46% of patients). Our outcome was defined as acute hospitalizations with an MS diagnosis registered as the cause of hospitalization.
Definition of exposure
Timing of influenza was examined in relation to hospitalizations with MS. Individuals were considered as having an influenza infection if they were diagnosed with influenza in primary care, or had a laboratory-confirmed diagnosis. We included information on influenza between 1st of January, 2008 (start of follow-up) to 15th of May, 2014 (end of last influenza season in the study). Pandemic influenza was defined as a diagnosis of influenza within the main wave of the H1N1 influenza pandemic, which in Norway lasted from 1st of October, 2009, to 31st of December, 2009 [16, 20]. Seasonal influenza was defined as a diagnosis of influenza during the influenza surveillance periods in Norway (September to May) for the period 2008–2014, excluding the pandemic season (from September 2009–May 2010). The season starting September 2014 was not included due to short follow-up time.
The pandemic vaccination campaign began on 19th of October, 2009 and vaccine was offered to the whole population free of charge. About 40% of the population was vaccinated and more than 97% of pandemic vaccinations were administered before 31st of December, 2009 [21].
Statistical analysis
We applied the self-controlled case series (SCCS) method to estimate incidence rate ratios (IRRs) of acute hospitalization with MS diagnosis in various risk periods following a diagnosis of influenza infection or vaccination, compared with a background period [22]. This method uses information from cases only. The strength of this method is that patients serve as their own controls, thus confounding from all unmeasured factors that do not vary in the study period is eliminated. To avoid surveillance bias, only acute hospitalizations were included. We analysed person-time and timing of hospitalization according to defined time periods (0–1, 2–3, 4–6, 7–9, and 10–12 weeks after influenza infection and vaccination). This study included the influenza seasons from 1st January, 2008 until 15th of May, 2014. Individuals were followed until outcome of interest, death, emigration, or the end of study on 31st of December, 2014, whichever occurred first.
The SCCS approach was applied in four separate models. In model I, we estimated the IRR of acute hospitalization among MS patients following a diagnosis of influenza, including any type of influenza (any influenza seasons including the pandemic) in the period from 1st of January, 2008 through 15th of May, 2014. In model II, we estimated IRR of acute hospitalization among MS patients only during the main pandemic period (1st of October, 2009 through 31st of December, 2009). In model III, the IRR of acute hospitalization among patients was estimated following a diagnosis of seasonal influenza infection in the period from 1st of January, 2008 through 15st May, 2014, excluding the pandemic season (from September 2009–May 2010). In model IV, we estimated IRR of acute hospitalization following pandemic vaccination (1st of October, 2009 through 30th of April, 2014). IRR was estimated using conditional Poisson regression and adjusted for age at hospitalization.
In an effort to evaluate vaccine safety in patients using different types of MS medications, as suggested in previous studies [23, 24], we studied the risk of acute hospitalization among MS patients using interferon beta versus non-interferon beta medications. Only MS patients with available data on medication were included in this sub-analysis.
All analyses were performed by using the Stata 13 software (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.).
Results
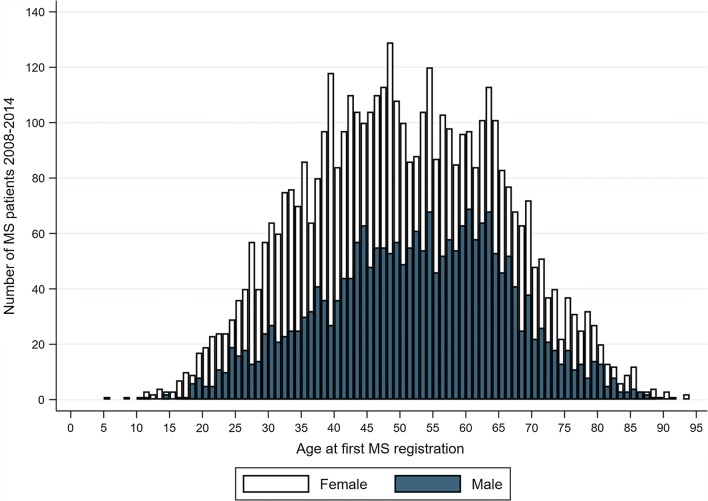
During 2008–2014, there were 6755 MS patients fulfilling the study criteria. Details on MS patients’ characteristics is summarized in Table 1. More women (67.1%) than men were registered with MS. Average age at first registration with an MS diagnosis in specialist care was 51.8 years among men and 49.9 years among women (Fig. 1). During the whole study period, an influenza diagnosis (seasonal and pandemic combined) was registered for 12.7% of MS patients, compared with 12.9% in the general population (Table 2). Overall, 60.7% of MS patients received the pandemic vaccine, 57.4% among men and 62.3% among women. In the general population (individuals without an MS diagnosis), 37.3% were vaccinated with the pandemic vaccine, 34.2% among men and 40.4% among women.
Table 1.
Characteristics of the patients identified with multiple sclerosis (first registration) by year of hospitalizations (2004–2014) in Norway
| Year of hospitalization among MS-patients | ||||||||
|---|---|---|---|---|---|---|---|---|
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | Total | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Sex | ||||||||
| Male | 572 (25.6) | 398 (17.8) | 330 (14.8) | 283 (12.7) | 247 (11.1) | 202 (9.0) | 204 (9.1) | 2236 (100) |
| Female | 1096 (24.3) | 827 (18.3) | 641 (14.2) | 573 (12.7) | 530 (11.7) | 435 (9.6) | 417 (9.2) | 4519 (100) |
| Year of birth | ||||||||
| 1915–1935 | 135 (41.0) | 70 (21.3) | 44 (13.4) | 33 (10.0) | 19 (5.8) | 17 (5.2) | 11 (3.3) | 329 (100) |
| 1936–1955 | 705 (29.3) | 435 (18.1) | 359 (14.9) | 265 (11.0) | 259 (10.8) | 201 (8.4) | 181 (7.5) | 2405 (100) |
| 1956–1975 | 674 (23.1) | 574 (19.6) | 441 (15.1) | 386 (13.2) | 335 (11.5) | 253 (8.7) | 260 (8.9) | 2923 (100) |
| 1976–1995 | 153 (14.3) | 146 (13.6) | 123 (11.5) | 168 (15.6) | 158 (14.7) | 161 (15.0) | 165 (15.4) | 1074 (100) |
| 1996–2007 | 1 (4.2) | 0 (0.0) | 4 (16.7) | 4 (16.7) | 6 (25.0) | 5 (20.8) | 4 (16.7) | 24 (100) |
| Total | 1688 (24.7) | 1255 (18.1) | 971 (14.4) | 856 (12.7) | 777 (11.5) | 673 (9.4) | 621 (9.2) | 6755 (100) |
An MS patient was defined as having at least one registration of an MS diagnosis registered in specialist care, combined with at least one dispensed MS medication. If lacking information on medication use, at least two registrations of MS diagnoses were required
Fig. 1.
Age distribution of MS patients in the study population for the period 2008–2014
Table 2.
Characteristics of the patients identified with Multiple Sclerosis (first registration) by influenza seasons and vaccination in Norway during 2008–2014
| Pandemic vaccination | Influenza | MS cases | |||
|---|---|---|---|---|---|
| N (%) | Overalla | Pandemicb | Seasonalc | N (%) | |
| N (%) | N (%) | N (%) | |||
| Total | 4099 (60.7) | 857 (12.7) | 262 (3.9) | 821 (12.6) | 6755 (100) |
| Sex | |||||
| Male | 1283 (57.4) | 213 (9.5) | 83 (3.7) | 203 (9.4) | 2236 (33.1) |
| Female | 2816 (62.3) | 644 (14.3) | 179 (4.0) | 618 (14.2) | 4519 (66.9) |
| Year of birth | |||||
| 1915–1935 | 136 (41.3) | 8 (2.4) | 13 (4.0) | 8 (2.6) | 329 (4.9) |
| 1936–1955 | 1576 (65.5) | 178 (7.4) | 84 (3.5) | 168 (7.3) | 2405 (35.6) |
| 1956–1975 | 1831 (62.6) | 417 (14.3) | 132 (4.5) | 401 (14.4) | 2923 (43.3) |
| 1976–1995 | 542 (50.5) | 251 (23.4) | 33 (3.1) | 245 (23.5) | 1074 (15.9) |
| 1996–2007 | 14 (58.3) | 3 (12.5) | 0 | 3 (12.5) | 24 (0.4) |
An MS patient was defined as having at least one registration of an MS diagnosis registered in specialist care, combined with at least one dispensed MS medication. If lacking information on medication use, at least two registrations of MS diagnoses were required
aAll influenza diagnoses for the period 2008–2014
bAn influenza diagnosis during the main pandemic wave in Norway (October 1st, 2009 to December 31st, 2009)
cAll influenza diagnoses for the period 2008–2014 excluding the pandemic period
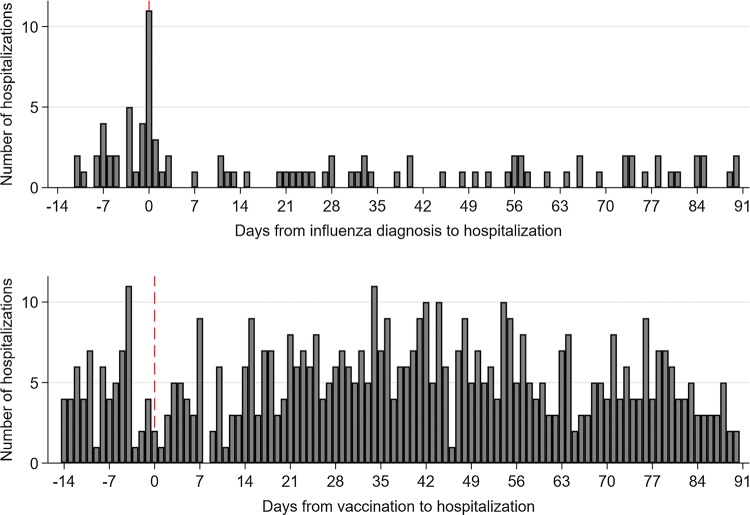
Hospitalizations for MS registered at the same day as the diagnosis of influenza infection was high compared with the number of hospitalizations on subsequent days. There were no differences in the patterns of hospitalizations before and following pandemic vaccinations (Fig. 2). The IRR of acute hospitalization within 1 week after an influenza infection (model I) was elevated when compared with the other time periods (IRR 3.4, 95% CI 2.4–4.8) (Table 3). Similar findings were observed following pandemic influenza (IRR 5.6, 95% CI 2.7–11.3) (model II) and following seasonal influenza (IRR: 4.8, 95% CI 3.1–7.4) (model III). Our analysis evaluating risk of acute hospitalization following pandemic vaccination did not indicate any association at any time point among MS patients (IRR of first week post vaccination: 0.7, 95% CI 0.5–1.0) (model IV).
Fig. 2.
Number of days from influenza diagnosis (top panel), and from day of vaccination (bottom panel) to an acute hospitalization, in 14 days prior and 90 days post infection or vaccination
Table 3.
Incidence rate ratio (IRR) of acute hospitalization among multiple sclerosis (MS) patients (N = 6755) in defined risk windows (period with risk of hospitalization) from January 1st, 2008 through December 31st, 2014, with associated 95% confidence interval (CI); estimated by the self-controlled case series method
| Risk window | Influenza | Vaccination | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (Model I)a | Pandemic influenza (model II)b | Seasonal influenza (model III)c | Pandemic vaccination (model IV)d | |||||
| Number of hospitalizations | IRR (95% CI) | Number of hospitalizations | IRR (95% CI) | Number of hospitalizations | IRR (95% CI) | Number of hospitalizations | IRR (95% CI) | |
| Background period | 6912 | – | 6363 | – | 6935 | – | 6985 | – |
| 2 weeks pre-exposure | 16 | 0.9 (0.5–1.4) | 5 | 1.7 (0.7–4.2) | 9 | 1.1 (0.6–2.1) | 51 | 0.6 (0.5–0.8) |
| 0–1 week post exposure | 32 | 3.4 (2.4–4.8) | 8 | 5.6 (2.7–11.3) | 21 | 4.8 (3.1–7.4) | 32 | 0.7 (0.5–1.0) |
| 2–3 weeks post exposure | 18 | 0.9 (0.6–1.5) | 3 | 1.0 (0.3–3.1) | 11 | 1.2 (0.7–2.2) | 80 | 0.9 (0.7–1.1) |
| 4–6 weeks post exposure | 16 | 0.6 (0.4–1.0) | 4 | 0.9 (0.4–2.6) | 7 | 0.6 (0.3–1.2) | 114 | 0.9 (0.8–1.1) |
| 7–9 weeks post exposure | 24 | 0.9 (0.6–1.4) | 2 | 0.5 (0.1–1.9) | 15 | 1.3 (0.8–2.1) | 123 | 1.0 (0.8–1.2) |
| 10–12 weeks post exposure | 22 | 0.8 (0.5–1.2) | 3 | 0.7 (0.2–2.2) | 10 | 0.8 (0.4–1.5) | 131 | 1.0 (0.9–1.2) |
Analysis were adjusted by age at hospitalization
aIncludes all influenza diagnoses occurring during 2008–2014
bIncludes diagnoses with pandemic influenza (between October 1st, 2009 through December 31st, 2009)
cInclude diagnoses with seasonal influenza occurring during 2008–2014, excluding the pandemic influenza (September 2009–May 2010)
dIRRs were calculated following pandemic vaccination
In a sub-analysis on risk of acute hospitalization, we compared MS patients using interferon beta (2592 patients) with those using non-interferon beta medications (2743 patients). The sub-analyses yielded similar results as the main analyses.
Discussion
In this nationwide registry-based study from Norway with individual level data. We observed an increased risk of acute hospitalizations with MS within the first week after a diagnosis of seasonal or pandemic influenza infection. Pandemic vaccination was not associated with risk of acute hospitalization among MS patients.
Strengths and weaknesses
A major strength of the current study was the availability of information from several independent health registries for the complete Norwegian population of more than 5.2 million individuals. Further, data on hospitalization, influenza infection and vaccination were prospectively collected and recorded independently in separate databases, by different procedures and systems, which minimizes the risk of bias due to differential reporting and selection. In Norway, the public health care system is financed through governmental funding and all hospitalizations are free of charge. MS is a serious condition which is most likely treated in hospitals, meaning that information on hospitalization is recorded in the national registries. Norwegian health registries and databases have high quality with mandatory reporting and minimal loss to follow-up. Another strength of the study was the SCCS method accounting for any factor or characteristic that is not measured but remains constant over the observation period, such as genetics, gender, socio-economic status, and lifestyle related factors [25].
A limitation of this registry-based study is that MS diagnoses were not validated. Diagnosis of MS is a prolonged and challenging process and some patients could be misdiagnosed [26]. However, a recent validation study on individuals residing in Nordland County in Norway and registered with MS in NPR examined the medical record of these individuals and reported that 91.5% of patients had a confirmed MS diagnosis [27]. We believe that our inclusion criteria for MS patients (patients with at least two registered diagnoses in specialist care or combination of information on diagnoses in specialist care with information on use of MS medications) provided a highly reliable identification of MS patients. It is likely that we mostly included patients with the relapsing–remitting form with or without secondary progression. Primary progressive MS cases accounts for 10–15% of the overall population with MS, and these patients may be missed or under-represented in our study [6, 28].
Another weakness of the study is under-reporting of influenza infections in primary care, as only those seeking medical attention for influenza are registered. It has been estimated that around 5–10% of the population is infected with influenza during a regular season [29]. During the 2009 pandemic outbreak less than 4% of the general Norwegian population were diagnosed with influenza by a primary care physician [15, 30], however the clinical attack rate of influenza was estimated to be approximately 25–30.0% [21]. In our study sample, the overall proportion in the general population with an influenza diagnosis during the 2009 pandemic outbreak was 2.5%. Among MS patients, 4% were diagnosed with pandemic influenza. This supports that MS patients were more likely to seek medical help when having influenza, possibly due to the seriousness of MS disease and the potential risks associated with having an influenza infection. It could also reflect that MS patients are more susceptible to influenza than healthy persons [2]. However, as those with very mild influenza or milder symptoms of MS may have been less likely to seek health care, our results may reflect associations with more severe influenza symptoms, or associations in patients with more severe MS. Another possible weakness is the potential misclassification when using influenza diagnoses from primary care. However, our results remained similar to the main analysis when including only cases with laboratory confirmed pandemic influenza.
Comparison to the literature
In accordance with previous studies, there was an increased risk of acute hospitalization among MS patients after an influenza diagnosis [6, 7, 9, 10], and no association between pandemic vaccination and risk of acute hospitalization was found [11–13]. More than six thousand MS patients were included in our study, which to our knowledge, is the largest study of seasonal and pandemic influenza infection among MS patients. Although we could not identify true MS relapses in our data, we believe studying acute hospitalizations may provide an indication for worsening MS-related symptoms and relapses. Previous studies did not focus on influenza infection and mostly combined several types of infections [7–9], and these were based on substantially smaller number of patients, and more selected study populations.
In previous studies, worsening MS-related symptoms among patients has been reported following infection [2, 7, 8]. Disease-modifying therapies alter the immune function and may potentially increase the risk of infections [2]. A population-based Swedish study reported higher hospital admission due to infections, including influenza. However, type of influenza and time from infection to hospitalization was not reported [4]. A questionnaire study among 233 Dutch MS patients, focused on effect of influenza illness and exacerbation of MS symptoms and reported a significantly higher rate of exacerbations among MS patients with influenza compared with those without influenza [6]. Our results support that within the first week following influenza infection, the risk of acute hospitalization was high among MS patients. A longitudinal study in 73 patients with relapsing–remitting multiple sclerosis assessed the contribution of upper airway infections to the risk of exacerbations [7]. They reported a significantly increased risk of exacerbation 2 weeks before until 5 weeks after the onset of a clinical infection (rate ratio 2.1), which also is in accordance with our study.
Risk of relapse after influenza vaccination (seasonal and pandemic) has been addressed in previous studies which are comparable to ours and no change in risk has been reported [11–13]. We observed that a higher proportion of MS patients were vaccinated against pandemic influenza compared with the general population. MS patients were defined as a risk group and were especially recommended pandemic vaccination. In our study, we did not observe any change in risk of acute hospitalization following pandemic vaccination which is in concordance with previous findings on safety of vaccination. Vaccination is considered as a possible trigger for autoimmune disease activity such as MS, however, previous studies as well as the current indicate that immunization is safe and may prevent influenza infection and potential complications. For MS patients, type and timing of vaccine (with inactivated influenza virus or live attenuated) combined with MS specific medications (interferon-beta versus non-interferon-beta medications) should be considered carefully [9]. In our sub-analyses, stratified by interferon-beta and non-interferon-beta medications, we found no differences in risk estimates related to vaccination.
Studies of influenza vaccination and risk of relapse have mainly been based on case reports and questionnaires. Such study designs are prone to selection/recall bias, and small study samples which may undermine the internal and external validity of a study. We acknowledge that underreporting of influenza infection is highly likely in our study. However, we utilized timing of events in the SCCS model in which patients serve as their own control, which enable us to address effects of both seasonal and pandemic influenza. SCCS models, to our knowledge, have not previously been applied in this context. Careful consideration of vaccination, taking the patient’s personal history into account, could help preventing infections and worsening of MS-related symptoms.
Conclusion
In this nationwide registry-based study we found an increased risk for acute hospitalization among MS patients within the first week after influenza diagnosis. Influenza infections may cause worsening of MS-related symptoms and trigger new relapses and should be prevented when possible. We observed no excess risk of acute hospitalization following pandemic vaccination. Our study indicates that MS patients could benefit from influenza vaccination, as reducing the risk of influenza infections could prevent worsening of MS-related symptoms and possibly new relapses.
Funding
The work was supported by the Norwegian Institute of Public Health and by the Research Council of Norway through its Centres of Excellence funding scheme [grant number 262700]. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of this manuscript.
Compliance with ethical standards
Conflict of interest
The authors have no conflict of interest.
Ethical approval
The study was approved by the Regional Committee for Medical and Health Research Ethics, South-East Region, Norway, and the Norwegian Data Protection Authority.
Footnotes
Data from the Norwegian Patient Registry has been used in this publication. The interpretation and reporting of these data are the sole responsibility of the authors, and no endorsement by the Norwegian Patient Registry is intended nor should be inferred.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Williamson EM, Berger JR. Infection risk in patients on multiple sclerosis therapeutics. CNS Drugs. 2015;29(3):229–244. doi: 10.1007/s40263-015-0226-2. [DOI] [PubMed] [Google Scholar]
- 3.Marrie RA, Elliott L, Marriott J, et al. Dramatically changing rates and reasons for hospitalization in multiple sclerosis. Neurology. 2014;83(10):929–937. doi: 10.1212/WNL.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montgomery S, Hillert J, Bahmanyar S. Hospital admission due to infections in multiple sclerosis patients. Eur J Neurol. 2013;20(8):1153–1160. doi: 10.1111/ene.12130. [DOI] [PubMed] [Google Scholar]
- 5.Evans C, Kingwell E, Zhu F, Oger J, Zhao Y, Tremlett H. Hospital admissions and MS: temporal trends and patient characteristics. Am J Manag Care. 2012;18(11):735–742. [PubMed] [Google Scholar]
- 6.De Keyser J, Zwanikken C, Boon M. Effects of influenza vaccination and influenza illness on exacerbations in multiple sclerosis. J Neurol Sci. 1998;159(1):51–53. doi: 10.1016/S0022-510X(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 7.Buljevac D, Flach HZ, Hop WC, et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. 2002;125(Pt 5):952–960. doi: 10.1093/brain/awf098. [DOI] [PubMed] [Google Scholar]
- 8.Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. 2006;67(4):652–659. doi: 10.1212/01.wnl.0000233834.09743.3b. [DOI] [PubMed] [Google Scholar]
- 9.Rutschmann OT, McCrory DC, Matchar DB. Immunization panel of the multiple sclerosis council for clinical practice guidelines. Neurology. 2002;59(12):1837–1843. doi: 10.1212/WNL.59.12.1837. [DOI] [PubMed] [Google Scholar]
- 10.Oikonen M, Laaksonen M, Aalto V, et al. Temporal relationship between environmental influenza A and Epstein–Barr viral infections and high multiple sclerosis relapse occurrence. Mult Scler. 2011;17(6):672–680. doi: 10.1177/1352458510394397. [DOI] [PubMed] [Google Scholar]
- 11.Auriel E, Gadoth A, Regev K, Karni A. Seasonal and H1N1v influenza vaccines in MS: safety and compliance. J Neurol Sci. 2012;314(1–2):102–103. doi: 10.1016/j.jns.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Mailand MT, Frederiksen JL. Vaccines and multiple sclerosis: a systematic review. J Neurol. 2016 doi: 10.1007/s00415-016-8263-4. [DOI] [PubMed] [Google Scholar]
- 13.Farez MF, Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J Neurol. 2011;258(7):1197–1206. doi: 10.1007/s00415-011-5984-2. [DOI] [PubMed] [Google Scholar]
- 14.Bakken IJ, Gystad SO, Christensen OO, et al. Comparison of data from the Norwegian Patient Register and the Cancer Registry of Norway. Tidsskr Nor Laegeforen. 2012;132(11):1336–1340. doi: 10.4045/tidsskr.11.1099. [DOI] [PubMed] [Google Scholar]
- 15.Ghaderi S, Stordal K, Gunnes N, Bakken IJ, Magnus P, Haberg SE. Encephalitis after influenza and vaccination: a nationwide population-based registry study from Norway. Int J Epidemiol. 2017;46(5):1618–1626. doi: 10.1093/ije/dyx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haberg SE, Trogstad L, Gunnes N, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med. 2013;368(4):333–340. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Health TNIoP. Norwegian Surveillance System for Communicable Diseases (MSIS). The Norwegian Instiute of Public Health, Oslo, Norway. https://www.fhi.no/en/health-in-norway/health-registries/norwegian-surveillance-system-for-communicable-diseases-msis/. Accessed 15 July 2016.
- 18.Trogstad L, Ung G, Hagerup-Jenssen M, Cappelen I, Haugen IL, Feiring B. The Norwegian immunisation register—SYSVAK. Euro Surveill. 2012;17(16):20147. [PubMed] [Google Scholar]
- 19.Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sorensen HT. The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol. 2010;106(2):86–94. doi: 10.1111/j.1742-7843.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- 20.Trogstad L, Bakken IJ, Gunnes N, et al. Narcolepsy and hypersomnia in Norwegian children and young adults following the influenza A(H1N1) 2009 pandemic. Vaccine. 2017;35(15):1879–1885. doi: 10.1016/j.vaccine.2017.02.053. [DOI] [PubMed] [Google Scholar]
- 21.Blasio BF, Iversen BG, Tomba GS. Effect of vaccines and antivirals during the major 2009 A(H1N1) pandemic wave in Norway—and the influence of vaccination timing. PLoS ONE. 2012;7(1):e30018. doi: 10.1371/journal.pone.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitaker HJ, Farrington CP, Spiessens B, Musonda P. Tutorial in biostatistics: the self-controlled case series method. Stat Med. 2006;25(10):1768–1797. doi: 10.1002/sim.2302. [DOI] [PubMed] [Google Scholar]
- 23.Metze C, Winkelmann A, Loebermann M, et al. Immunogenicity and predictors of response to a single dose trivalent seasonal influenza vaccine in multiple sclerosis patients receiving disease-modifying therapies. CNS Neurosci Ther. 2018 doi: 10.1111/cns.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baghbanian SM. Influenza vaccination in patients with multiple sclerosis is possible with some considerations. Iran J Neurol. 2016;15(2):109–110. [PMC free article] [PubMed] [Google Scholar]
- 25.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354:i4515. doi: 10.1136/bmj.i4515. [DOI] [PubMed] [Google Scholar]
- 26.Solomon AJ, Klein EP, Bourdette D. “Undiagnosing” multiple sclerosis: the challenge of misdiagnosis in MS. Neurology. 2012;78(24):1986–1991. doi: 10.1212/WNL.0b013e318259e1b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benjaminsen E, Myhr KM, Grytten N, Alstadhaug KB. Validation of the multiple sclerosis diagnosis in the Norwegian Patient Registry. Brain Behav. 2019;9(11):e01422. doi: 10.1002/brb3.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 29.Norwegian Institute of Public Health O. Influenza—Fact sheet about seasonal influenza 2016. https://www.fhi.no/en/id/influensa/seasonal-influenza/influenza—fact-sheet-about-season/. Accessed 1 July 2019.
- 30.Hauge SH, Bakken IJ, de Blasio BF, Haberg SE. Burden of medically attended influenza in Norway 2008–2017. Influenza Other Respir Viruses. 2019;13(3):240–247. doi: 10.1111/irv.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]