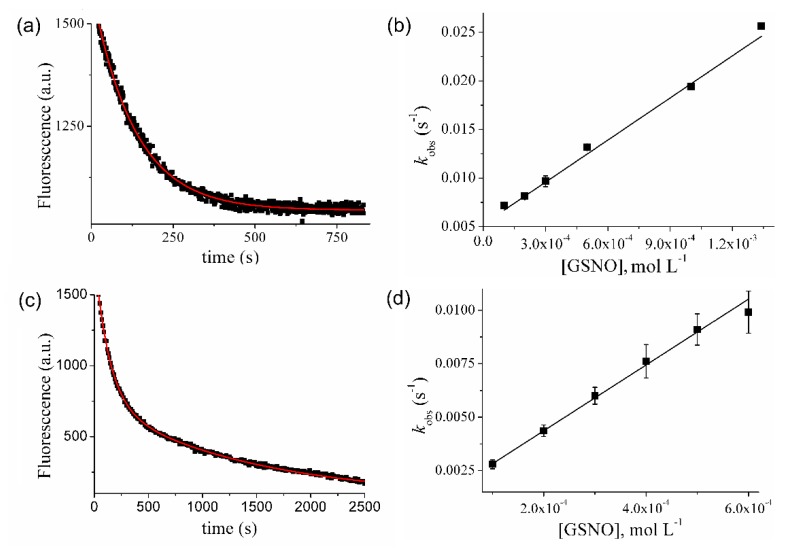
Figure 2.
Kinetics of GSNO-mediated nitrosation of Prx1C83SC173S and of Prx1. (a) Representative kinetics of reduced Prx1C83SC173S (5 µM) S-nitrosation by GSNO (400 μM) (λexc. = 280 nm; λem. = 330 nm). The red trace corresponds to the fitting of the data to a single-exponential function. (b) Determination of the second-order rate constant of the reaction of Prx1 catalytic cysteine (Cys52) with GSNO. Pseudo-first-order rate constants (kobs) were plotted against GSNO concentration and the second-order rate constant obtained from the slope. (c) Representative kinetics of reduced Prx1 (5 µM) S-nitrosation by GSNO (400 μM). The red trace corresponds to the fitting of the data to a double-exponential function. (d) Determination of the second-order rate constant of the reaction of wild type Prx1 Cys83 with GSNO. Pseudo-first-order rate constants (kobs) for the second-exponential fluorescence decay were obtained by fixing the first-exponential fluorescence decay to the kobs value obtained from the Prx1C83SC173S and GSNO reaction. Then, the kobs values were plotted against GSNO concentration and the second-order rate constant obtained from the slope. All the experiments were performed using a Hitachi F-2500 fluorimeter in phosphate buffer (50 mM) containing DTPA (0.1 mM), pH 7.4, at 25 °C.