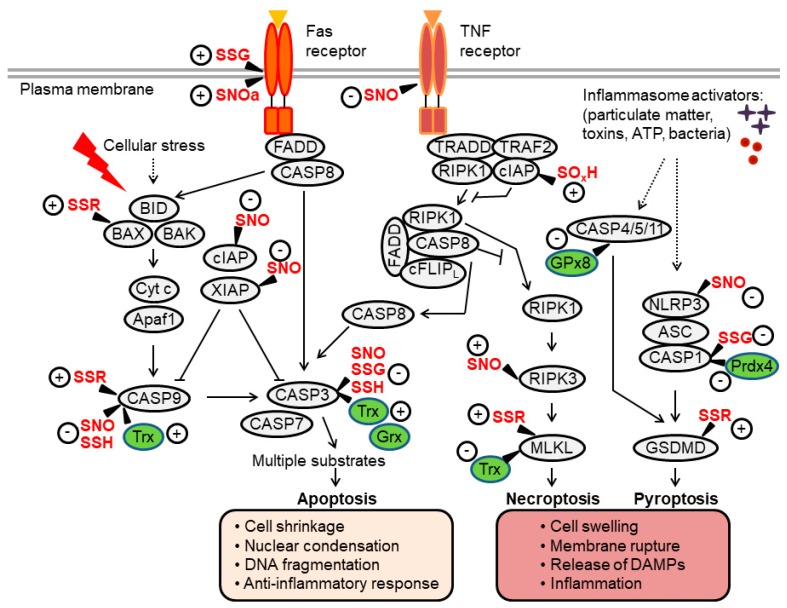
Figure 2.
Overview of apoptosis, necroptosis and pyroptosis pathways and their regulation by thiol redox modifications. Extrinsic apoptosis is mediated by the Fas-associated death domain (FADD)-caspase-8 axis, which activates executioner caspases (caspase-3, -7). Intrinsic apoptosis involves mitochondrial outer membrane permeabilization, a process regulated by B-cell lymphoma (BCL)-2-family proteins (such as BID, BAX and BAK). This leads to the formation of the apoptosome (a complex of cytochrome c (Cyt c) and apoptotic protease-activating factor-1 (Apaf-1), which facilitates activation of caspase-9 upstream of caspase-3/7. Activation of the tumor necrosis factor (TNF) receptor can induce apoptosis through a signaling cascade that activates caspase-8. Under conditions of caspase-8 inhibition, a protein complex consisting of receptor-interacting kinase (RIPK)1/3 and mixed lineage kinase-like (MLKL) is formed, promoting the execution of necroptotic cell death. In inflammatory cells such as macrophages, pathogens and danger signals promote the assembly of inflammasome complexes, which activate caspase-1 or, in some settings, mouse caspase-11 (human caspase-4/-5). These inflammatory caspases cleave gasdermin D (GSDMD) to induce pyroptosis. Proteins directly regulated by thiol modifications or redox enzymes (thioredoxin [Trx], glutaredoxin [Grx], glutathione peroxidase [GPx]) are highlighted, where “+” and “-“ indicate positive and negative effects on protein activity, respectively (see the main text for further details). Through these thiol redox switches, oxidants and Trx/GSH systems influence regulated cell death (RCD) processes and the crosstalk between them. SNO, nitrosylation; SOH, sulfenylation; SSG, glutathionylation; SSR, disulfide formation; SOxH, oxidation or hyperoxidation.