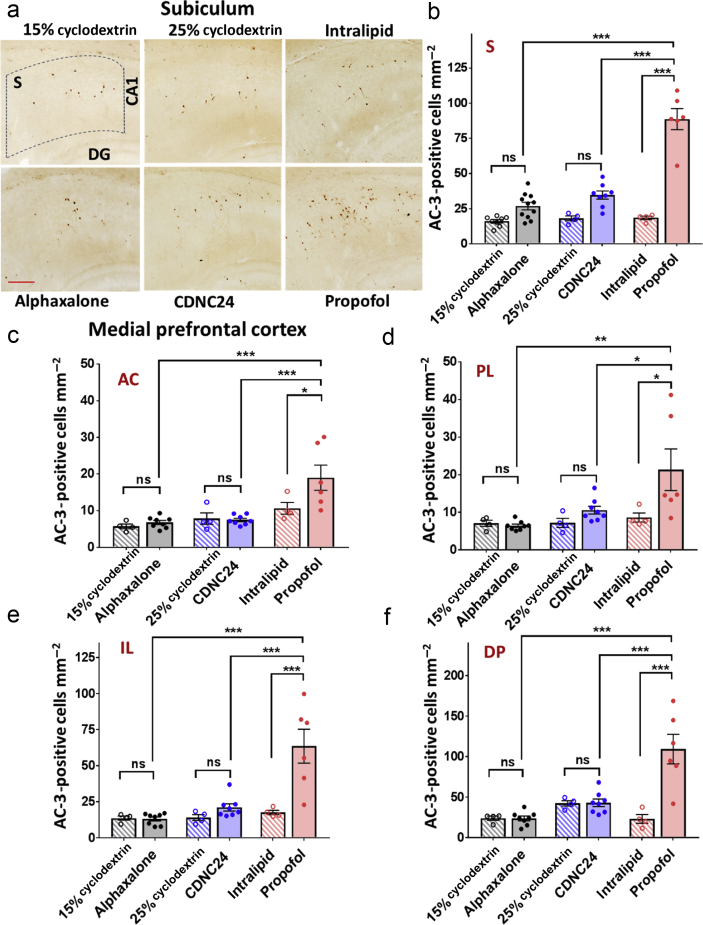
Figure 2.
Alphaxalone and CDNC24 do not cause developmental neuroapoptosis in the subiculum and medial prefrontal cortex of postnatal Day 7 rat pups. (a) Representative images of activated caspase-3 (AC-3) immunostaining in the subiculum after six i. p. injections of alphaxalone, CDNC24, or propofol, and cyclodextrin 15%, cyclodextrin 25%, or intralipid as controls, respectively, are depicted in the panel. Scale bar is 200 μm. (b) Bar graphs showing the number of AC-3-positive cells mm−2 in subiculum after six i. p. injections of alphaxalone (filled grey bar), CDNC24 (filled blue bar), or propofol (filled red bar), and cyclodextrin 15% (patterned grey bar), cyclodextrin 25% (patterned blue bar), or intralipid (patterned red bar) as their respective vehicle controls. Propofol caused a significant increase in the number of AC-3-positive cells compared with intralipid (4.8-fold; one-way analysis of variance [anova] followed by Tukey's post hoc test: F5,35=52.27; P<0.001; ∗∗∗P<0.001), alphaxalone or CDNC24 (both ∗∗∗P<0.001). Neither neurosteroid caused an increase in the number of apoptotic cells in the immature subiculum when compared with their respective controls (P>0.05; ns, not significant). (c–f) Bar graphs showing the number of AC-3-positive cells mm−2 in (c) anterior cingulate (AC), (d) prelimbic (PL), (e) infralimbic (IL), and (f) dorsal peduncular (DP) subregions of medial prefrontal cortex (mPFC) after six i. p. injections of alphaxalone (filled grey bar), CDNC24 (filled blue bar), or propofol (filled red bar), and cyclodextrin 15% (patterned grey bar), cyclodextrin 25% (patterned blue bar), or intralipid (patterned red bar) as their vehicle controls, respectively. In the AC subregion, propofol caused a 1.8-fold increase in the number of AC-3 labelled cells in comparison with intralipid control (one-way anova followed by Tukey's post hoc test: F5,28=8.82; P<0.001; ∗P=0.033), whilst alphaxalone and CDNC24 did not show a neurotoxic effect compared with their respective controls. In PL, a 2.5-fold increase in the number of apoptotic cells was detected with propofol compared with intralipid control (one-way anova followed by Tukey's post hoc test: F5,28=5.09; P=0.002; ∗P=0.033). No difference was seen between neurosteroids and their controls (cyclodextrin 15% and cyclodextrin 25% groups). In IL, propofol increased the number of AC-3-positive cells 3.6-fold, significantly above the level detected with both neurosteroids that remained in the control level (one-way anova followed by Tukey's post hoc test: F5,28=13.60; P<0.001; ∗∗∗P<0.001). In DP, propofol caused a 4.7-fold increase in comparison with intralipid. The neurosteroids showed no neurotoxic effect when compared with controls, with the number of AC-3 labelled cells significantly lower than propofol (one-way anova followed by Tukey's post hoc test: F5,28=14.63; P<0.001; ∗∗∗P<0.001). In all mPFC subregions, the number of AC-3-positive cells in the propofol group was significantly higher than in either alphaxalone or CDNC24 group (all comparisons at least ∗P<0.05). All data are presented as mean (standard error of the mean [sem]) (n=4–11 rats per each data point). Representative images of AC-3-positive cells in subregions of mPFC can be found in Supplementary Figure S2. Exact values of mean (sem) numbers of AC-3-positive cells in each treatment and all regions presented in graphs (b–f) can be found in Supplementary Table S1.