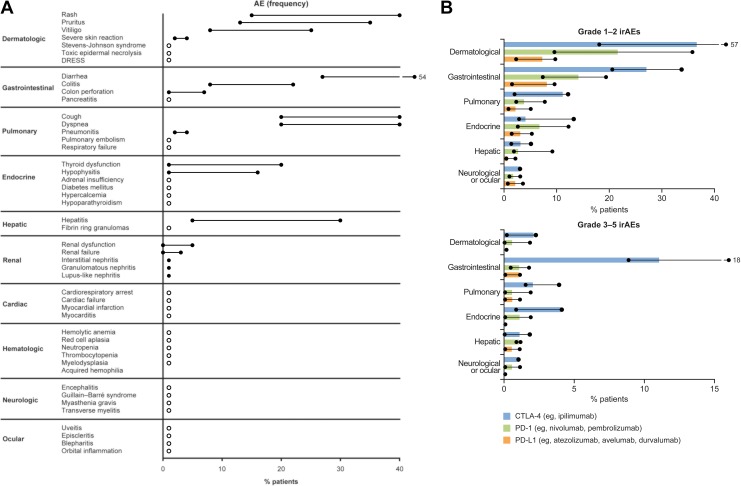
Figure 1.
Frequency of irAEs for ICI monotherapya by (A) organ system (range) and (B) class of ICI and gradeb (median and range). Circles and lines represent the range of frequency. White circles in (A) represent <1%. Solid bars in (B) represent the median frequency. aMonotherapies include nivolumab, pembrolizumab, and ipilimumab. birAE grading according to National Cancer Institute Common Terminology Criteria for Adverse Events. (A) Data collated from prospective and retrospective phase 2 and 3 clinical studies summarized and cited in the study by Pardoll et al, Buchbinder et al, Tarhini et al, Haanen et al, and Puzanov et al.9–11,13,14 The number of patients in clinical studies ranged from 14 to 1685. (B) Adapted from Michot et al, where the phase 2 and 3 clinical studies from which the data were summarized are cited.12 The number of patients in these studies ranged from 135 to 799. CTLA-4, cytotoxic T lymphocyte antigen-4; DRESS, drug rash with eosinophilia and systemic symptoms; GI, gastrointestinal; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; PD-1, programmed death-1; PD-L1, programmed death-ligand 1.