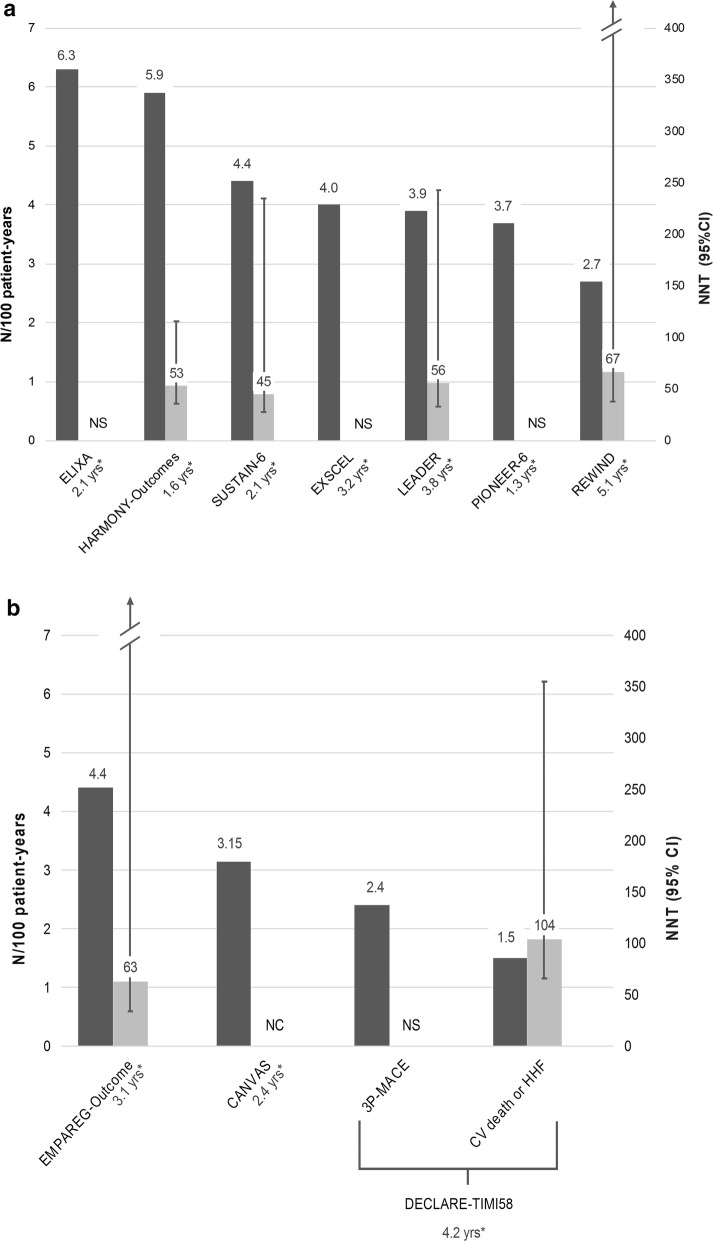
Fig. 3.
Graphic illustration of annual placebo primary outcome rates and associated NNTs in GLP-1 RA (a) and SGLT-2i (b) CVOTs. GLP-1 RA: Glucagon Like Peptide-1 receptor agonists; SGLT-2i: Sodium-Glucose Co-Transporter-2 inhibitors; NNT: Number Needed to Treat; CVOTs: cardiovascular outcomes trials; N/100 patient-years: number per 100 patient-years; 95% CI: 95% confidence interval; CV: cardiovascular; HHF: hospitalization for heart failure; NS: not significant; NC: not calculable because required data for calculation were not available in the publication paper or supplementary appendix. *median study follow-up in years; Primary outcome was a 3-points MACE (Major Adverse Cardiovascular Events) for all studies, except ELIXA (4-points MACE) and DECLARE-TIMI58 (co-primary endpoint: 3P-MACE and CV death or HHF); Dark grey bars represent annual placebo primary outcome rates; Light grey bars represent NNTs with 95% CI; regarding data from the REWIND and EMPAREG-Outcome studies, a vertical arrow and 2 slash signs were used to represent the upper limit of their respective 95% confidence intervals for NNTs on a sensible scale