Abstract
Purpose
Sepsis survivors have a higher risk of rehospitalisation and of long-term mortality. We assessed the rate, diagnosis, and independent predictors for rehospitalisation in adult sepsis survivors.
Methods
We searched for non-randomized studies and randomized clinical trials in MEDLINE, Cochrane Library, Web of Science, and EMBASE (OVID interface, 1992–October 2019). The search strategy used controlled vocabulary terms and text words for sepsis and hospital readmission, limited to humans, and English language. Two authors independently selected studies and extracted data using predefined criteria and data extraction forms.
Results
The literature search identified 12,544 records. Among 56 studies (36 full and 20 conference abstracts) that met our inclusion criteria, all were non-randomised studies. Studies most often report 30-day rehospitalisation rate (mean 21.4%, 95% confidence interval [CI] 17.6–25.4%; N = 36 studies reporting 6,729,617 patients). The mean (95%CI) rehospitalisation rates increased from 9.3% (8.3–10.3%) by 7 days to 39.0% (22.0–59.4%) by 365 days. Infection was the most common rehospitalisation diagnosis. Risk factors that increased the rehospitalisation risk in sepsis survivors were generic characteristics such as older age, male, comorbidities, non-elective admissions, hospitalisation prior to index sepsis admission, and sepsis characteristics such as infection and illness severity, with hospital characteristics showing inconsistent associations. The overall certainty of evidence was moderate for rehospitalisation rates and low for risk factors.
Conclusions
Rehospitalisation events are common in sepsis survivors, with one in five rehospitalisation events occurring within 30 days of hospital discharge following an index sepsis admission. The generic and sepsis-specific characteristics at index sepsis admission are commonly reported risk factors for rehospitalisation.
Registration
PROSPERO CRD 42016039257, registered on 14-06-2016.
Electronic supplementary material
The online version of this article (10.1007/s00134-019-05908-3) contains supplementary material, which is available to authorized users.
Keywords: Sepsis, Rehospitalisation, Risk factors, Competing risk
Take-home message
|
Nearly 50% of sepsis survivors have at least one unplanned rehospitalisation by 1 year following hospital discharge from their index sepsis admission. Many of the risk factors for this rehospitalisation are acute illness characteristics at index sepsis admission such as age, comorbidities, site of infection, and illness severity. |
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1] and is a global health priority [2]. In cohort studies, mainly from critically ill adults from high-income countries, sepsis diagnosis is increasing, and short-term mortality is improving [3–5]. This epidemiology pattern results in increasing numbers of sepsis survivors, defined as patients who survive a sepsis-related hospitalisation. Among the numerous long-term ill health consequences observed in sepsis survivors, increased risk of rehospitalisation and long-term mortality [6], when compared with non-sepsis hospitalisations and age-sex matched general population, are major challenges [7, 8]. Importantly, a proportion of this increased risk of rehospitalisation in sepsis survivors may be modifiable [9].
Similar to the challenge of determining causation with the reported associations between sepsis and long-term mortality [10], the risk of rehospitalisation in sepsis survivors may be sepsis-related or may reflect an event that is common to anyone who survives a hospitalisation episode [11]. Thus, we hypothesised that this rehospitalisation risk in sepsis survivors may vary with both patient characteristics and health care system characteristics [12, 13]. Therefore, understanding the independent and potentially modifiable risk factors that contribute towards this additional rehospitalisation risk seen in sepsis survivors would inform future interventional trials aimed at reducing this risk.
In this context, the first aim of our systematic review was to assess the rehospitalisation rate, the associated major rehospitalisation diagnoses, and the excess risk of all-cause rehospitalisation due to sepsis in sepsis survivors using studies reporting comparator populations. The second aim was to assess the independent risk factors for rehospitalisation using studies that report design features or analytic approach to control confounding [14, 15], such as use of comparator populations, matching, restriction, stratification, and regression. The third aim was to assess how studies handled the competing risk of mortality in sepsis survivors, when rehospitalisation events are studied as the outcome of interest [10, 16, 17]. This competing risk problem may be more common in health care settings where community-level end-of-life or hospice care is more prevalent [18, 19].
Methods
Our study conforms to the MOOSE checklist for systematic reviews of observational studies [20].
Information sources
Using the OVID interface, we searched for non-randomized studies and randomized clinical trials (RCTs) published since 1992 in the following databases: MEDLINE (including in-process and non-indexed citations), Cochrane Library and its associated databases (including Database of Abstracts of Reviews of Effects (DARE), Web of Science, and EMBASE. The search strategy used controlled vocabulary terms and text words for sepsis and hospital readmission, and the search set was limited to humans and English language. Subject headings were exploded and mapped to the appropriate controlled vocabulary terms. The year 1992 was chosen to coincide with the year of publication of the first consensus sepsis definitions [21]. The full electronic search strategy for MEDLINE is presented in electronic supplementary material (eTable-1) and modified for other databases and registered with the International prospective register of systematic reviews (PROSPERO CRD 42016039257). The initial literature search was on 31st March 2017 and was updated on 5th October 2019.
Study selection
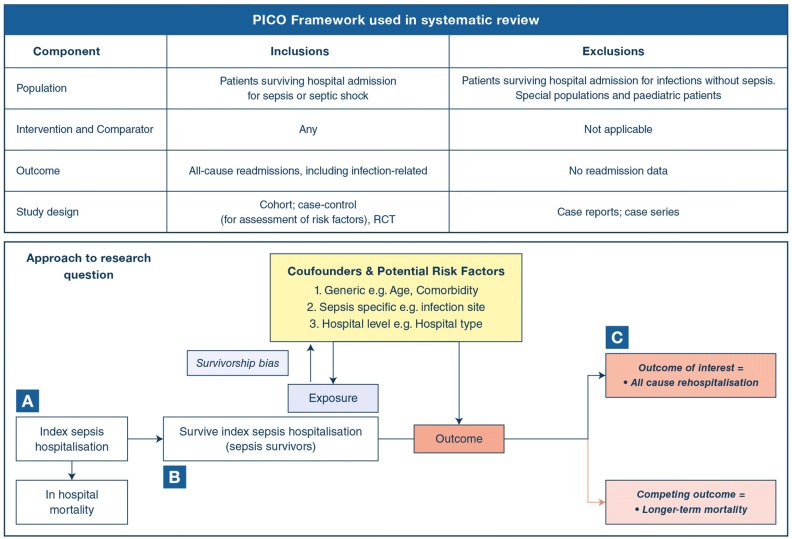
Two reviewers (RS, MSH) independently screened citations for those reporting all-cause rehospitalisation for sepsis survivor populations in the title or abstract; the full text of any citation considered potentially relevant by either reviewer was retrieved. Eligible studies had a cohort, case–control, or Randomised-Controlled Trial (RCT) design; enrolled hospital survivors of an admission for sepsis; and reported all-cause readmission. An eligible RCT would have enrolled sepsis survivors and examined any intervention. The PICO framework for study selection is reported in Fig. 1.
Fig. 1.
PICO summary and approach to research question. The principal exposure was surviving an index sepsis-related hospitalisation (sepsis survivors). The outcome of interest was all-cause rehospitalisation, which will be affected by a survivorship bias in the observed associations, as sepsis survivors are likely to be healthier than patients who die during the sepsis-related hospitalisation and b bias from competing risk as sepsis survivors also have a long-term risk of mortality. Shorter follow-up times in rehospitalisation studies preclude observation of outcome of interest (i.e., censored outcomes). A = Sepsis cohort starting from their index admission which may have greater risk of survivorship bias; B = Ideal cohort to address the research question; and C = Re-hospitalised survivor cohort all patients have the outcome of interest and there is limited understanding of the competing risk issue. Studies with non-sepsis controls provide an estimate the excess risk of rehospitalisation that is unique to sepsis [10, 87]
For inclusion into the systematic review, sepsis was defined as infection-related organ dysfunction [1] managed in hospital setting and includes studies that used the equivalent terminology of sepsis, severe sepsis, and septic shock [1, 22]. We excluded studies restricted to children and to special populations such as those with retroviral disease, cancer, and other immune-compromised states, although studies that enrolled these special populations as part of a more general cohort were eligible for inclusion. We also excluded studies enrolling survivors of uncomplicated infections, such as pneumonia, without referring to organ dysfunction or to International Classification of Diseases (ICD) codes for sepsis, severe sepsis, or septic shock in their index sepsis case definitions. Prior to finalising the literature strategy in October 2016, infection-related rehospitalisation was revised to a secondary outcome; the primary outcome was considered as all-cause rehospitalisation. However, this point was only updated in the PROSPERO record prior to submission for peer review. At the screening stage, we considered any study design and included review articles and editorials accompanying original relevant studies. We also screened reference lists of included studies, related review articles, and editorials.
Data collection and validity assessment
When two or more studies were identified that reported data from the same patient cohort, the most relevant article was chosen by consensus (JW, RS, MSH). The most relevant article was defined as the most recent full manuscript, if the data from the same patient cohort were reported as abstract or as an earlier full manuscript. Three authors (JW, RS, MSH) extracted data from the included studies and issues of uncertainty were resolved by consensus. We included full manuscripts and conference abstracts for estimating the timing and rate of rehospitalisation and only the full manuscripts for assessing rehospitalisation diagnoses, independent risk factors, and the competing risk problem. From each of the included studies, we extracted data on study design, number of patients, duration of follow-up, handling of loss during follow-up, description of index sepsis admission, rehospitalisation events, rehospitalisation diagnoses, independent risk factors for rehospitalisation, and approach to competing risk of long-term mortality [8]. We classified risk factors as generic, sepsis-related, or hospital-related according to a previously used framework [6, 8].
Assessment of methodological quality
For studies reported as full-text manuscripts, study quality was assessed using domains from the modified Newcastle Ottawa Score (NOS) checklist [23]. These included domains of patient selection (cohort data source for representativeness of exposed cohort, selection of non-exposed cohort, exposure ascertainment using sepsis definitions or International Classification of Diseases codes), minimum duration of follow-up for outcome to occur was defined as 30 days, assessment of confounding (use of comparator populations, matching, restriction, stratification, and regression), and comparability using non-sepsis controls and outcome (outcome assessment, length, and adequacy of follow-up). The independent risk factors for rehospitalisation were identified from studies that used regression models to account for confounders. We assessed the overall certainty of evidence using the GRADE framework [24], considering the risk of bias of included studies (as described above), inconsistency, imprecision, indirectness, and publication bias.
Statistics
Our conceptual approach is summarised in Fig. 1. The primary outcome of interest was all-cause rehospitalisation events in sepsis survivors following an index episode of sepsis, at follow-up time points as reported in studies. We recategorized the rehospitalisation-associated risk factors into generic, sepsis-specific, and hospital-level factors. We included age, sex, ethnicity, rural or urban residence, socioeconomic status, educational attainment, and comorbidity as generic risk factors. We included infection, septic shock status, acute illness severity including physiological disturbance, organ support, and organ dysfunction as sepsis-specific risk factors. We included hospital location (urban versus rural), university status (university-affiliated vs not), and other reported descriptions as hospital-level risk factors. We provide a descriptive comparison of risk factors included in analysis between studies and those risk factors identified as increasing the risk of rehospitalisation in sepsis survivors between studies. We performed random effect metanalysis of proportions (using metaprop package) [25] of cumulative rehospitalizations at 7, 30, 90, 180, and 365 days; between-study heterogeneity was assessed using I2, which is the percentage of between-study variation due to heterogeneity rather than chance, with values of 25%, 50%, and 75% indicating low, moderate, and high heterogeneity, respectively [26]. We assessed small-study effects using Egger’s test for 7-, 30-, 90-, 180-, and 365-day proportions, when there were at least ten studies at a given time point. All analyses were done using Stata/MP 14.2 StataCorp College Station, Texas 77845, USA.
Results
Study selection
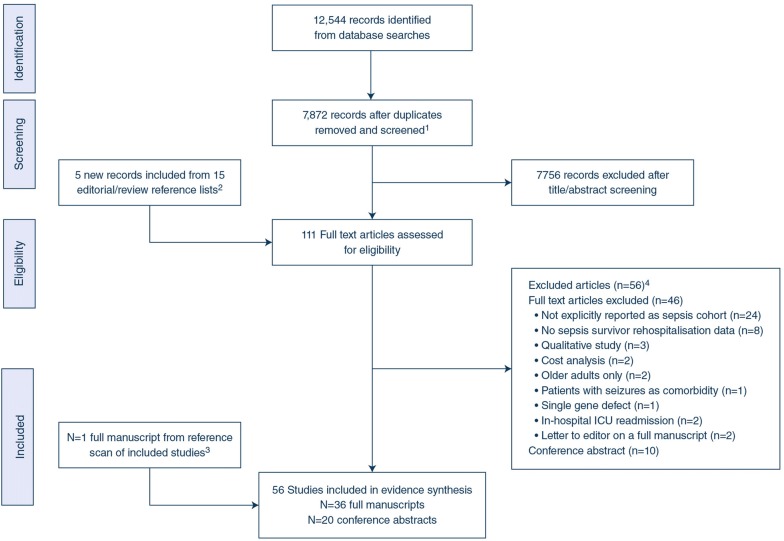
The bibliographic database search identified 12,544 records. After exclusion of duplicates, we identified 7,872 records for screening. Following screening, 111 records were considered eligible for full-text evaluation. Based on full-text evaluation, we excluded 56 records (reasons for exclusion reported in Fig. 2 and the excluded papers are referenced in eMethods-1). We included one study from the reference scan of included full manuscripts, resulting in 56 unique studies that met our inclusion criteria for the systematic review (36 full manuscripts [9, 12, 13, 27–59] and 20 conference abstracts [60–79], (Fig. 2). All studies were observational; we did not identify any RCTs enrolling sepsis survivors.
Fig. 2.
Flow diagram showing literature search and results. Flow of information through the different phases of our systematic review recorded PRISMA reporting guidelines. We identified 5184 records from searching MEDLINE, 3810 records from searching EMBASE, 474 records from searching Ovid other/ non-indexed database, and 2039 records from searching the Cochrane library. We identified a further 1037 records from searching the Web of Science database (using TOPIC (septic*) and TOPIC (readmission*) = 244; TOPIC (sepsis*) and TOPIC (readmission*) = 793). This literature search resulted in a total of 12,544 records for our systematic review. 1At screening stage, we included original articles, review articles, and editorials. 2Reference list from editorial and review articles that met the screening criteria were included for full-text review. 3One full manuscript from reference list scan of the 36 included full manuscripts. 4Excluded studies are listed in ESM
Methodological quality of included studies
Our study selection criteria ensured that all 36 studies had the exposure of interest, sepsis, thereby avoiding differential exposure measurement that contributes towards risk of bias [9, 12, 13, 27–59]. All 36 studies met the minimum follow-up duration of 30 days [9, 12, 13, 27–59], that we considered as adequate for outcome of interest to occur. Ten studies report a sepsis cohort starting from their index admission [27, 35, 37, 41, 44, 46, 50, 51, 53, 55], twelve studies report a sepsis survivor cohort [9, 12, 30–32, 36, 45, 47, 52, 56, 58, 59], and four report a rehospitalisation cohort [28, 34, 40, 42]. Ten were single-centre studies [28, 37, 40, 44, 47, 50, 51, 55, 56, 59] with greater risk of bias compared to 21 studies [9, 12, 13, 27, 29-31, 33, 35, 36, 38, 39, 41, 42, 45, 48, 49, 53, 54, 57] that used large multi-centre databases with greater generalizability. Five studies that use notes review for outcome assessment [28, 37, 40, 51, 55] have a greater risk of ascertainment bias, compared to studies that use record linkage outcome assessment. The primary outcome was all-cause rehospitalisation in 21 studies [9, 12, 13, 28-30, 35, 36, 38, 39, 41, 42, 45, 47, 48, 50-52, 56, 57, 59]. Confounders for rehospitalisation risk factors were addressed with regression models in seventeen [12, 29, 33, 35, 36, 38–41, 45, 47, 50–52, 55, 56, 59] including competing risk models in two [38, 41], matching in two [9, 49], stratification in one [50], and restriction in one [33]. Twenty-one studies were of low risk of bias and 15 studies were at moderate risk of bias for the primary outcome of rehospitalisation risk, as per modified Newcastle–Ottawa criteria (Table 1).
Table 1.
Quality assessment and overall risk of bias of original research articles included in the systematic review
| Study ID | Cohort data source | Cohort description | Ascertainment of sepsis exposure | Minimum 30-day follow-up | Follow-up method and outcome assessment | Was primary study outcome all-cause rehospitalisation | Confounder assessment for rehospitalisation risk factors in sepsis survivors | Non-sepsis comparisons | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| Braun et al. [27] | MC-large | Sepsis cohort | Yes | Yes | Record linkage | No | Not assessed | No | Low |
| Cakir et al. [28] | SC | Re-hospitalised cohort | Yes | Yes | Notes review | Yes | Not assessed | Yes | Moderate |
| Chang et al. [29] | MC-large | Sepsis and non-sepsis patients in cohort | Yes | Yes | Record linkage | Yes | Regression | Yes | Low |
| Deb P et al. [30] | MC-large | Sepsis survivors | Yes | Yes | Record linkage | Yes | Regression | No | Low |
| DeMerle et al. [31] | MC-large | Sepsis survivors | Yes | Yes | Record linkage | No | Not assessed | No | Low |
| DeMerle et al. [32] | SC | Sepsis survivors | Yes | Yes | Notes review | No | Not assessed | No | Low |
| Dick et al. [33] | MC-large | Sepsis and non-sepsis patients | Yes | Yes | Record linkage | No | Not assessed | Yes | Low |
| Dietz et al. [34] | MC | Re-hospitalised cohort | Yes | Yes | EHR | No | Not assessed | Yes | Low |
| Donnelly et al. [12] | MC-large | Sepsis survivors | Yes | Yes | Record linkage | Yes | Regression | No | Low |
| Gadre et al. [35] | MC-large | Sepsis cohort | Yes | Yes | Record linkage | Yes | Regression | No | Low |
| Goodwin et al. [36] | MC-large | Sepsis survivors | Yes | Yes | Record linkage | Yes | Regression | No | Low |
| Guirgis et al. [37] | SC | Sepsis cohort | Yes | Yes | Notes review | No | Not assessed | No | Moderate |
| Hua et al. [38] | MC-large | Sepsis and non-sepsis patients in cohort | Yes | Yes | Record linkage | Yes | Regression; competing risk model | Yes | Low |
| Jones et al. [39] | MC-large | Sepsis and non-sepsis patients in cohort | Yes | Yes | Record linkage | Yes | Regression | Yes | Low |
| Kim et al. [40] | SC | Re-hospitalised cohort | Yes | Yes | Notes review | No | Regression | No | Moderate |
| Liu et al. [41] | MC-large | Sepsis cohort | Yes | Yes | Record linkage | Yes | Regression; competing risk model | No | Low |
| Mayr et al. [42] | MC-large | Re-hospitalised cohort | Yes | Yes | Record linkage | Yes | Not assessed | Yes | Low |
| Meyer et al. [43] | MC | Sepsis and non-sepsis patients in cohort | Yes | Yes | Record linkage | No | Not assessed | Yes | Low |
| Nkemdirim Okere et al. [44] | SC | Sepsis cohort | Yes | Yes | Record linkage | No | Restriction; not assessed | No | Moderate |
| Norman et al. [45] | MC-large | Sepsis survivors | Yes | Yes | Record linkage | Yes | Regression | No | Low |
| Nsutebu et al. [46] | MC | Sepsis cohort | Yes | Yes | Notes review | No | Not assessed | No | Moderate |
| Ortego et al. [47] | SC | Sepsis survivors | Yes | Yes | Record linkage | Yes | Regression | No | Low |
| Prescott et al. [49] | MC-large | Sepsis and non-sepsis patients in cohort | Yes | Yes | Record linkage | No | Matching | Yes | Low |
| Prescott et al. [9] | MC-Large | Sepsis and non-sepsis patients in cohort | Yes | Yes | Record linkage | Yes | Matching | Yes | Low |
| Prescott et al. [48] | MC-large | Sepsis and non-sepsis patients in cohort | Yes | Yes | Record linkage | Yes | Not assessed | Yes | Moderate |
| Prescott et al. [13] | MC-large | Sepsis survivors | Yes | Yes | Record linkage | Yes | Not assessed | No | Moderate |
| Schnegelsberg et al. [50] | SC | Sepsis cohort | Yes | Yes | Record linkage | Yes | Stratification | No | Moderate |
| Singh et al. [51] | SC | Sepsis cohort | Yes | Yes | Notes review | Yes | Regression | No | Moderate |
| Sun A et al. [52] | MC | Sepsis survivors | Yes | Yes | Notes review | Yes | Regression | No | Moderate |
| Sutton et al. [53] | MC-Large | Sepsis cohort | Yes | Yes | Record linkage | No | Not assessed | No | Moderate |
| Vashi et al. [54] | MC-large | Sepsis and non-sepsis patients in cohort | Yes | Yes | Record linkage | No | Not assessed | Yes | Low |
| Wang et al. [55] | SC | Sepsis | Yes | Yes | Notes review | No | Regression | Yes | Moderate |
| Weinreich et al. [56] | SC | Sepsis survivors | Yes | Yes | Hospital EHR | Yes | Regression | No | Moderate |
| Wong EL et al. [57] | MC-large | Sepsis and non-sepsis patients in cohort | Yes | Yes | Record linkage | Yes | Not assessed | Yes | Moderate |
| Yende et al. [58] | MC | Sepsis survivors | Yes | Yes | Prospective cohort | No | Not assessed | No | Low |
| Zilberberg et al. [59] | SC | Sepsis survivors | Yes | Yes | Hospital EHR | Yes | Regression | No | Moderate |
The risk of bias was assessed on patient selection, ascertainment of exposure, and ascertainment of outcome domains using a modified Newcastle Ottawa Score (NOS) quality assessment checklist [23]. These domains account for bias with ascertainment, generalisability, measurement of exposure, measurement of risk factors, and selection. Comparability domain of NOS assessed whether excess risk of rehospitalisation in sepsis survivors was quantified and how confounders were considered during study design or analysis with techniques such as matching, restriction or regression models. Outcome domain of NOS assessed bias due to incomplete assessment of outcome or of competing risk outcomes such as mortality and due to censoring. Study-level risk of bias is then reported. Using this information, overall certainty of evidence was assessed as per GRADE system of assessment of evidence about prognosis (see main results) [24]
EHR electronic health record, MC multi-centre, SC single-centre
Primary outcome (rate of all-cause rehospitalisation)
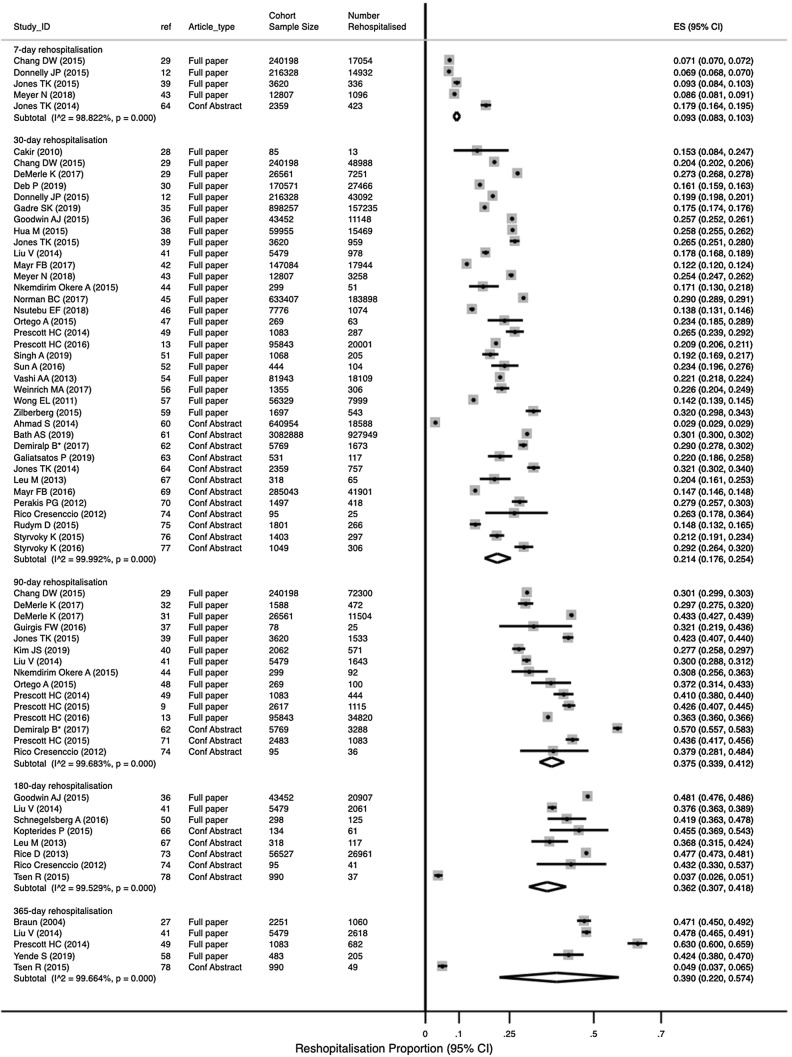
Studies most often reported the 30-day rehospitalisation events in a sepsis survivor population. The mean rehospitalisation proportion (95% CI) at 30 days was 21.4% (17.6%, 25.4%; N = 36 studies reporting 6,729,617 patients; Fig. 3), at 7 days was 9.3% (8.3%, 10.3%; N = 5 studies reporting 475,312 patients), at 90 days was 38.1% (34.3%, 42.0%; N = 14 studies, 388,044 patients), at 180 days was 36.2% (30.7%, 41.8%; N = 7 studies, 107,293 patients), and at 365 days was 39.0% (22.0%; 57.4%; N = 5 studies, 10,286 patients). All estimates had high heterogeneity. We did not observe any small-study effects (eTable-1). Two studies that use competing risk models [38, 41] also had similar 30-day rehospitalisation rates (eFigure-1; test for heterogeneity between groups p = 0.08). There were no differences in 30-day rehospitalisation rates by risk of bias (eFigure-2; test for heterogeneity between groups p = 0.33). In studies with non-sepsis comparator populations, the 30-day rehospitalisation proportions in sepsis survivors were reported as either comparable to congestive heart failure and acute myocardial infarction [9, 29, 54], or much higher than these and other similar acute medical conditions [33, 34, 39, 42, 55, 57]. The median (IQR) acute mortality among sepsis survivors who were re-hospitalised was 6.6% (4.6%, 8.7%; N = 8 studies) [12, 13, 29, 34, 36, 38, 39, 57].
Fig. 3.
Rate and timing of rehospitalisation. Random effect meta-analysis of proportions by rehospitalisation interval reported in all studies
Diagnosis at rehospitalisation
Studies that report rehospitalisation diagnoses in sepsis survivors grouped these diagnoses using clinical classification software (CCS) codes [29, 35, 38, 41], ambulatory care sensitive conditions codes (ACSCs) [9], or other customised categories [13, 47, 52] (Table 2). The relationship between infection at index sepsis admission and the infection diagnosis at rehospitalisation was reported in one study as recurrent or unresolved in nearly 50% of cases [52], often secondary to opportunistic pathogens like Pseudomonas aeruginosa and Candida species in another study [55], and same site as index sepsis admission in 68% of rehospitalisation events in another study [32]. Infection-related rehospitalisation was the most common rehospitalisation event in sepsis survivors. The median (IQR) 30-day event rate was 49.3% (38.0%, 61.2%) of the all rehospitalisation events in ten studies [12, 29, 34–36, 38, 47, 51, 52, 56], with similar proportions reported at 90 days [37, 40] and 365 days [41]. Between one-third and two-thirds of rehospitalisation episodes in sepsis survivors were coded as sepsis [29, 32, 52] (Table 2).
Table 2.
Rehospitalization diagnosis according to diagnostic classification scheme used in selected studies
| CCS criteria | Liu V et al. [41] N = 4310a Liberal at 1-year |
Chang DW et al. [29] N = 240,198a At 30 days |
Gadre SK et al. [35] N = 1,030,335 At 30 days |
Top-10 ACSCs | Prescott H et al. [9] N = 2617b |
Other | Prescott H et al. [13] N = 16,844 (2011 data at 90 days) |
Ortego A et al. [47] N = 63a At 30 days |
Sun et al. [52] N = 104a At 30 days |
Hua M et al. [38] N = 44,051 At 30 days |
|---|---|---|---|---|---|---|---|---|---|---|
| Infectious | 42.7% | 59.3% | 42.2% | Sepsis | 6.4% | Infections | 14.3% | 46% | 69.2% | 25.5% |
| Circulatory | 13.6% | 6.8% | 8.7% | CHF | 5.5% | Cardiovascular and thromboembolic | 7.4% | 17.5% | 12.5% | 29.5% |
| Respiratory | 9% | 12.8% | 7.8% | Pneumonia | 3.5% | Acute Kidney injury or Genitourinary | 4.4% | 6.4% | 5.8% | 2.7% |
| Digestive | 6.6% | 3.1% | 9.6% | Acute renal failure | 3.3% | Complications of devices | 2.7% | 3.2% | 3.8% | 4.7% |
| Injury and poisoning | 8.9% | Rehabilitation | 2.8% | Other | 4.8% | 8.6% | ||||
| Genitourinary | 2.6% | 5% | 5% | Acute Respiratory failure | 2.5% | Complication of procedure | 2.8% | 15.3% | ||
| Endocrine and metabolic | 4.6% | Complications | 2% | Respiratory | 6.6% | 6.4% | ||||
| Neoplastic | 4.1% | COPD Exacerbation | 1.9% | Fluid and electrolyte disorder | 2.6% | |||||
| Dermatologic | 0.4% | Aspiration pneumonitis | 1.8% | Related to comorbid condition | 22.2% | |||||
| Musculoskeletal | 1.7% | UTI | 1.7% | Diabetes Mellitus complications | 2.7% | |||||
| Hematologic | 1.9% | Fluid or electrolyte disorder | Gastrointestinal | 2.5% | ||||||
| Nervous system | 1.6% | |||||||||
| All others | 1.2% | 13.9% |
CCS Clinical classification software diagnostic categories, ASCS ambulatory care sensitive conditions, CHF congestive heart failure, COPD chronic obstructive pulmonary disease, UTI urinary tract infections
Independent risk factors for all-cause rehospitalisation
Among the 15 studies that identify independent risk factors for rehospitalisation events in sepsis survivors [12, 29, 30, 35, 36, 38-41, 45, 51, 52, 55, 56, 59], most analysed all-cause 30-day rehospitalisation as the outcome and two studies report independent risk factors for infection-related rehospitalisation [40, 55] (Table 3). Generic characteristics consistently highlighted as predictors for increased risk of rehospitalisation were increasing age, male sex, presence of one or comorbidities determined using either Charlson or Elixhauser comorbidity indices, non-white race, non-elective admissions, pre-index admission hospitalisation, and increased length of hospitalisation during index sepsis admission. Risk of rehospitalisation in sepsis survivors was increased when the discharge location was not to home following the index sepsis admission [13, 30, 34-36, 38, 51].
Table 3.
Summary of full manuscripts included in the systematic review and risk factors for increased risk of rehospitalisation in studies reporting regression models
| Study ID and Country | Study characteristics | Regression model for the outcome as reported in studies | Risk factors associated with increased risk of rehospitalisation in studies reporting regression models for rehospitalisation outcomes and risk factors for primary outcome for individual studies | |||
|---|---|---|---|---|---|---|
| Data source and sample size (N =) | Study primary outcome | Generic | Sepsis-specific | Hospital and other characteristics | ||
|
Braun et al. [27] USA |
Administrative claims data (not Managed Medicare) N = 2,834 |
Hospital length of stay and health service costs due to admission with severe sepsis | No | Not applicable | Not applicable | Not applicable |
|
Cakir et al. [28] USA |
Single-centre community hospital data N = 5,206 |
30-day rehospitalisation with same diagnosis as index hospitalisation | No | Not applicable | Not applicable | Not applicable |
|
Chang et al. [29] USA |
Healthcare Cost and Utilisation Project data N = 240,198 sepsis patients |
All-cause 30-day readmission after hospitalisation with sepsis | Mixed-effects logistic regression for 30-day rehospitalisation |
Younger age Male Black or Native American Higher burden of comorbidities |
No independent associations reported |
Hospitals serving higher proportion of minorities; For profit hospitals University hospital; Urban residence; Lower income |
|
Deb et al. [30] USA |
Medicare data N = 170,571 |
30-day all-cause hospital readmission | Multinomial logit model of 30-day study outcome categories | Comorbidities; unplanned weight loss; ADL dependencies; | Organ dysfunction (referred to as severe sepsis) | Home health nursing assessment of risk; |
| DeMerle et al. [31] |
Veterans Affairs data N = 26,561 |
Days spent in a healthcare facility | No | Not applicable | Not applicable | Not applicable |
| DeMerle et al. [32] |
University of Michigan Health System N = 472 |
90-day infection-related rehospitalisation characteristics | No | Not applicable | Not applicable | Not applicable |
|
Dick et al. [33] USA |
Medicare data N = 17,537 |
Survival and healthcare utilization for five years following index admission with sepsis, pneumonia, CLABSI or VAP | No | Not applicable | Not applicable | Not applicable |
|
Dietz et al. [34] USA |
University of Pennsylvania Health System (UPHS) data; N = 17,716 |
In-hospital mortality or transition to hospice during 30-day readmissions | Mixed-effects logistic regression for In-hospital death, or transition to hospice during 30-day read- missions | Older age; Higher burden of comorbidities; Prior hospitalisations; Non-elective index admission |
Sepsis Presence of shock |
Discharge disposition not to home; Lower discharge; levels of haemoglobin; Lower Sodium concentrations; Higher discharge levels of RDW; Insurance status |
|
Donnelly et al. [12] USA |
University Health System Consortium (UHC) data; N = 216,328 | Unplanned 7- and 30-day readmission after hospitalisation with severe sepsis | Mixed-effects logistic regression for 30-day rehospitalisation |
Female Longer index admission length of stay Higher burden of comorbidities |
Digestive system infection sites based on ICD-9 codes | Institutions with higher sepsis case volume and lower ICU utilisation |
|
Gadre et al. [35] USA |
Healthcare Cost and Utilisation Project National Readmissions data; N = 1,030,335 | 30-day all-cause readmissions | Multivariable regression model with hospital as random effect | Comorbidities; Longer length of stay | No associations with shock or mechanical ventilation | Discharge to short/long-term facility; Lower socioeconomic status |
|
Goodwin et al. [36] USA |
Healthcare Cost and Utilisation Project data; N = 43,452 | 30-day readmission after hospitalisation with severe sepsis | Multivariable logistic regression for 30-day rehospitalisation |
Age < 80 years Male Black Medicare or Medicaid as primary payer Comorbidities |
Sepsis-specific effect lost significance once comorbidities were accounted |
Discharge disposition not to home Institutions with higher sepsis case volume Higher in-hospital sepsis mortality |
|
Guirgis et al. [37] USA |
University of Florida (UF) Health Jacksonville Emergency Department data; N = 110 | Long-term organ dysfunction in sepsis survivors | No | Not applicable | Not applicable | Not applicable |
|
Hua et al. [38] USA |
New York State-wide Planning and Research Cooperative System (SPARCS) data; N = 492,653 | 30-day readmission after critical illness | Competing risk regression for 30-day rehospitalisation |
Older age Longer index admission length of stay Higher burden of comorbidities including Dialysis dependence; Medicaid as primary payer |
Organ dysfunction (described as severe sepsis) |
Discharge disposition not to home Tracheostomy at index admission |
|
Jones et al. [39] USA |
University of Pennsylvania Health System (UPHS) data; N = 3,620 sepsis and 108,958 non-sepsis | 30-day all-cause readmission after hospitalisation with sepsis | Multivariable logistic regression for 30-day rehospitalisation |
Lower age; Hospitalisation in previous year non-elective index admission |
No independent associations reported |
Lower discharge levels of haemoglobin Higher discharge levels of RDW |
|
Kim et al. [40] Republic of Korea |
Asan Medical Centre data; N = 2062 | Risk factors of readmission due to sepsis caused by the “same organism” within 90 days of discharge | Stepwise multivariate regression to identify risk factors for individual pathogen | Male sex lowers risk | Same site of infection; Gram-negative pathogen; UTI | No independent association reported |
|
Liu et al. [41] USA |
Kaiser Permanente Northern California data; N = 6,344 |
1-year rehospitalisation/ healthcare utilisation after hospitalisation with sepsis |
Competing risk regression for 30-day rehospitalisation | Older age; Higher burden of comorbidities; Longer index admission length of stay; | Illness severity at index admission | Requirement for ICU care |
|
Mayr et al. [42] USA |
2013 Nationwide readmission database; N = 147,084 sepsis patients | Unplanned 30-day readmission after sepsis hospitalisation | No | Not applicable | Not applicable | Not applicable |
|
Meyer et al. [43] USA |
University of Pennsylvania Health System (UPHS); N = 17,256 | Temporal trends in sepsis survivorship and hospital-based acute care use in sepsis survivors | No | Not applicable | Not applicable | Not applicable |
|
Nkemdirim Okere et al. [44] USA |
Ferris State University single-centre data; N = 661 | Length of stay; 30-, 60- and 90- day all-cause readmission after sepsis hospitalisation | No | Not applicable | Not applicable | Not applicable |
|
Norman et al. [45] USA |
Medicare database; N = 633,407 | All-cause 30-day readmission after hospitalisation with sepsis | Hospital-level risk-standardized 30-day all-cause readmission rates using regression models | No independent association reported | No independent associations reported | Teaching hospitals; Hospitals providing care for high proportion of underserved patients; Northeast USA geographic region |
|
Nsutebu et al. [46] England, UK |
Advancing Quality Sepsis data; N = 7,776 | The outcomes of interest were inpatient mortality, readmission within 30 days and hospitalisation longer than 10 days | No | Not applicable | Not applicable | Not applicable |
|
Ortego et al. [47] USA |
University of Pennsylvania Health System (UPHS); N = 997 | All-cause hospital readmission/ED visits within 30 days of discharge after hospitalisation with septic shock | Multivariable logistic regression for 30-day rehospitalisation |
Malignancy as comorbidity Length of stay greater than 4 days |
No independent associations reported | Recent hospitalisation within 30 days |
|
Prescott et al. [49] USA |
US Health and Retirement Study Data; N = 16,772 participants | Use of inpatient facilities (hospitals; long-term acute care hospitals; skilled nursing facilities) in the year following discharge after sepsis hospitalisation | No | Not applicable | Not applicable | Not applicable |
|
Prescott et al. [9] USA |
US Health and Retirement Study Data linked with Medicare claims data; N = 2,617 sepsis and 2,617 matched non-sepsis | 90-day readmission diagnoses after hospitalisation with severe sepsis compared to matched non-sepsis cohorts | No | Not applicable | Not applicable | Not applicable |
| Prescott et al. [48] | US Health and Retirement Study Data linked with Medicare claims data; N = 10,996 participants | Severe sepsis in 90 days following hospital discharge | No | Not applicable | Not applicable | Not applicable |
|
Prescott et al. [13] USA |
USA Veterans Affairs Database | 90-day all-cause readmission | hierarchical logistic regression with patients nested within hospitals for all-cause readmissions | Age | No independent associations reported | Discharge to nursing facility |
|
Schnegelsberg et al. [50] Denmark |
Aarhus University Hospital, Denmark sepsis research database; N = 387 | 30- and 180-day mortality; unplanned 180-day readmission after sepsis hospitalisation | Cox models adjusted for sex, comorbidity and SAPS II score for readmission or death | No independent association reported | No independent associations reported | Living alone |
|
Singh et al. [51] USA |
Saint Vincent Hospital data; N = 1,297 | 30-day unplanned readmissions | Multivariable logistic regression for 30-day readmissions | Prior hospitalisation in preceding year; | No independent associations reported | Discharge disposition to short-term rehab facility; Nursing home; Lower discharge haemoglobin |
|
Sun et al. [52] USA |
University of Pennsylvania Health System (UPHS) data; N = 444 | Unplanned 30-day readmission after hospitalisation with sepsis | Multivariable logistic regression for 30-day rehospitalisation | Prior hospitalisation before index sepsis episode | No independent associations reported | Use of Total parenteral nutrition; Longer duration of antibiotics; Lower discharge haemoglobin |
|
Sutton et al. [53] USA |
Healthcare Cost and Utilisation Project and State Inpatient database; N = 267,000 in 2005 | Trends in sepsis admissions and readmissions 2005—2010 | No | Not applicable | Not applicable | Not applicable |
|
Vashi et al. [54] USA |
Healthcare Cost and Utilization Project state inpatient and Emergency Department databases; N = 81,943 sepsis | ED visits (not resulting in admission); hospital readmissions from any source; combined measure of ED visits and hospital readmission | No | Not applicable | Not applicable | Not applicable |
|
Wang et al. [55] USA |
West Los Angeles Veteran Affairs (VA) Healthcare Centre, N = 78 sepsis and 50 non-sepsis | Recurrent infections in first year following hospitalisation with sepsis | Independent-incremental models for recurrent infection events related rehospitalisation | Advanced age, Admission from nursing home; | No independent associations reported | Prolonged hospitalisation; presence of indwelling catheter |
|
Weinreich et al. [56] USA |
Texas Southwestern Medical Centre data; N = 1,355 sepsis | All-cause 30- day readmissions | Multivariate logistic regression was used to identify factors associated with 30-day readmissions | Comorbidities (Malignancy, renal disease and cirrhosis) | Bacteraemia during index sepsis admission; | Discharged with an indwelling vascular catheter |
|
Wong et al. [57] Hong Kong |
Hong Kong Hospital Authority Database; N = 337,694 | 30-day readmission after index hospitalisation with ten common medical conditions | No | Not applicable | Not applicable | Not applicable |
|
Yende et al. [58] USA |
Prospective Cohort Study; N = 483 | 1-year included all-cause and cause-specific readmissions and mortality | No | Not applicable | Not applicable | Not applicable |
|
Zilberberg et al. [59] USA |
Barnes-Jewish Hospital data; N = 1,697 | All-cause 30-day readmission after hospitalisation with severe sepsis or septic shock | Multivariable logistic regression for 30-day rehospitalisation | No independent association reported | Presence of ESBL or Bacteroides spp; Acute Kidney injury; UTI | No independent association reported |
USA United States of America, ADL activities of daily living, ED emergency department, RDW red cell distribution width, CLABSI Catheter-related blood stream infection, VAP ventilator-associated pneumonia, COPD chronic obstructive pulmonary disease, UTI urinary tract infections, ICU intensive care unit, ESBL extended spectrum beta-lactamase
Among the sepsis-specific characteristics at index admission, infection features, organ dysfunction, and illness severity were identified as risk factors for rehospitalisation, especially when assessed with competing risk regression models [38, 41]. The type of infecting pathogen at index admission did not significantly alter the risk of rehospitalisation, with the exception of extended spectrum beta-lactamase (ESBL) producing bacteria [59]. When risk factors for the same pathogen as index sepsis admission for rehospitalisation were evaluated, same pathogen was identified only in 25% of rehospitalisation and the major risk factors for same pathogen rehospitalisation were Gram-negative bacteria, urosepsis, and same site of infection [40]. Similar to all-cause rehospitalisation, the risk factors for infection-related rehospitalisation were older age, prolonged hospitalisation, and nursing home residence [55]. In three studies, infection-related rehospitalisation episodes were associated with greater risk of death [32, 52, 55] when compared to non-infection-related hospitalisations.
Among hospital-level characteristics, risk of rehospitalisation in sepsis survivors varied significantly among hospitals in two studies [12, 29] and did not in one study [13]. The risk of rehospitalisation in sepsis survivors was higher in hospitals serving a higher proportion of minority population, in for profit hospitals compared with public/non-profit hospitals, in university or teaching hospitals vs. not, in hospitals that had higher sepsis case volume especially when associated with lower critical care usage, and in hospitals that had higher in-hospital mortality for sepsis index sepsis admissions [12, 29, 36, 45].
In studies with non-sepsis comparator populations, there were similarities in generic and hospital-level characteristics as risk factors for rehospitalisation in sepsis survivors and rehospitalisation seen with medical conditions such as congestive heart failure and acute myocardial infarction [29, 54]. Eight other studies report regression models that were not aimed at identifying rehospitalisation risk factors, but were designed to examine health care utilization [33], long-term organ dysfunction [37], effect of statins [44], subsequent severe sepsis following index all-cause hospitalization [48], variation in patterns of rehospitalization in sepsis survivors [13], additional risk of socioeconomic status in sepsis [50], and risk of sepsis compared to non-sepsis hospitalizations [55, 57].
Overall certainty of evidence
For the primary outcome of all-cause rehospitalisation, the certainty of evidence is moderate, based on low risk of bias in the majority of studies reporting 30-day rehospitalisation. We did not rate down further for imprecision or inconsistency, because confidence intervals around risks of rehospitalisation were reasonably narrow and compatible with clinically important risks. Studies generally had broad inclusion criteria representative of the exposure of interest, sepsis, and, therefore, provided direct evidence. There was no evidence of publication bias. For rehospitalisation risk factors, the certainty of evidence is low due to inconsistency in risk factor definitions, imprecision in strengths of association, and risk of bias in many studies due to lack of competing risk models.
Discussion
One in five sepsis survivors are re-hospitalised within 30 days of discharge from hospital. The cumulative proportion of sepsis survivors re-hospitalised plateaus at 40% between 90 and 365 days, which may be related to competing risk of long-term deaths in sepsis survivors. Only two studies considered competing risk of long-term mortality when studying risk factors for rehospitalisation in sepsis survivors. The most common rehospitalisation diagnosis in sepsis survivors was infection. Uncertainties remain as to whether this represents a new infection or recurring infection from the index sepsis admission. Independent risk factors of rehospitalisation were most often time-invariant predictors like older age, male sex, higher comorbidity burden, and hospitalisation immediately preceding the index sepsis admission, and discharge to non-home location. Among the sepsis-specific risk factors, gastrointestinal site of infection, infection with ESBL bacteria, increasing illness severity, and longer hospital length of stay during index admission increased the risk of rehospitalisation. Other characteristics that increased rehospitalisation risk were lower socioeconomic strata, lower discharge haemoglobin, use of total parenteral nutrition, and tracheostomy at index sepsis admission. Hospital-level characteristics such as for profit and university status and sepsis volumes also influenced the risk of rehospitalisation in sepsis survivors, albeit inconsistently.
Ours is first systematic review of the epidemiology of rehospitalisation events in the at-risk population of adult sepsis survivors, in the year following sepsis-related hospitalisation. We used a customized checklist to assess potential for bias in ascertainment of exposure, the outcome, and management of confounding. We limited the study population to adult sepsis survivors and the outcome to all-cause rehospitalisation. We report the rehospitalisation rates at different timepoints over the first year following sepsis survival. Our systematic review describes the excess risk of sepsis-related rehospitalisation up to 1 year, which will inform sample size estimations of trials focussing on sepsis survivors and when assessed within health care systems could inform follow-up care planning.
There are limitations to this systematic review. The rehospitalisation events and diagnoses were identified in most studies using data linkage. Although we excluded non-English language studies, this is unlikely to bias our results [80, 81]. We did not extract length of hospital stay data. The lack of any RCTs included in our systematic review may be related to the search strategy and screening criteria that focused on rehospitalisation events in sepsis survivors; we did not systematically examine all trials of septic patients to determine whether they reported rehospitalisation data. As the diagnostic codes are linked to hospital activity and remuneration, potential risk of bias from different coding practices cannot be ruled out. As our goal was to assess sepsis survivors’ risk of rehospitalisation, we excluded related conditions such as pneumonia [82] which could potentially have provided additional information on rehospitalisation risk factors. In a systematic review of that specifically addressed rehospitalisation after pneumonia, the 30-day all-cause rehospitalisation rates in 12 studies were 11.6%, which is lower than sepsis survivor rates which we observed [83]. Interestingly, the 1-year rehospitalisation rates following pneumonia was 46%, which is compared to the sepsis survivor rates which we observed [83]. Higher rehospitalisation rates following pneumonia were noted in US-based cohorts and the common reasons for rehospitalisation following pneumonia in the study were pneumonia (5.6%) and worsening of cardiac and pulmonary comorbidities [83]. We planned our study before guidelines for systematic reviews assessing prognostic factors were published [84]. Most studies have assessed rehospitalisation risk using previous definitions of sepsis or using ICD codes to identify sepsis. Thus, our study highlights that rehospitalisation epidemiology with a more recent sepsis survivor cohort, based on the updated sepsis definitions, would be a valuable addition to the literature [1, 85].
We categorised the rehospitalisation risk factors or predictors into generic, sepsis-specific, and hospital-level risk factors. We show that many of the risk factors for rehospitalisation are time-invariant predictors such as age, comorbidity, prior hospitalisation, site of infection at admission, and socioeconomic or deprivation status [29, 44, 50] such as insurance, lower income, urban residence, race, and education. These predictors have also been identified as risk factors for long-term mortality [6, 10] and are commonly available when sepsis survivors leave hospital. Therefore, a parsimonious prognostic risk score could be derived to stratify sepsis survivors based on their rehospitalisation risk, using their index sepsis admission variables. Our review also highlights the value of explicitly considering competing risk models in the analysis when assessing risk factors, as the cumulative rehospitalisation proportion plateaus after 90 days, potentially due to long-term mortality acting as competing event for rehospitalisation, especially in health care settings where community-level end-of-life or hospice care are more prevalent [18, 19].
Sepsis-specific characteristics such as features of infection and sepsis severity requiring critical care admission influenced this rehospitalisation risk [12, 34, 41, 59]. Furthermore, in our study, the most common rehospitalisation diagnosis in sepsis survivors was infection, which has been linked to microbiome alterations [48] and to immunological sequelae seen in sepsis survivors [58, 86]. Thus, understanding the microbiome and immunological status at critical care discharge will enable design of potential interventional trials in this population [8].
Hospital-level characteristics also influenced the risk of rehospitalisation in sepsis survivors, albeit inconsistently. Hospital sepsis case volume and critical care usage of sepsis patients influences subsequent rehospitalisation risk [36]. Furthermore, characteristics such as hospital size, university status, and serving a minority population appear to influence the risk of rehospitalisation. Thus, there is a need to assess the relative contributions of hospital- and patient-level predictors for this rehospitalisation risk, as reported for cardiovascular diseases [11]. These may provide opportunities for addressing this rehospitalisation problem with hospital-level quality-of-care interventions. For example, understanding how best to manage medical comorbidities in sepsis survivors [9] could alter the long-term risk of rehospitalisation and death.
Conclusions
One in five sepsis survivors are re-hospitalised within 30 days of discharge from hospital and this rehospitalisation risk is comparable with non-sepsis acute medical conditions. Generic patient characteristics (such as increasing age, comorbidity burden, and haemoglobin at discharge from hospital), sepsis-specific characteristics (such as type of infection), and hospital-level characteristics at their index sepsis admission influence this rehospitalisation risk. Our findings may inform the development of prognostic scores and the design of future interventional studies in this at-risk population of sepsis survivors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Manu Shankar-Hari is supported by the National Institute for Health Research Clinician Scientist Award (NIHR-CS-2016-16-011). Hallie Prescott was supported by US National Institutes of Health (K08 GM115859). This material is the result of work supported with resources and the use of facilities at the Ann ArborVA medical facility. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health, and social care. The views expressed in this publication are those of the author(s) and not necessarily those of the US Department of Veterans Affairs.
Author contributions
MSH conceived the study. MSH developed the search strategy and performed the literature search. MSH/RS/JW/NA did the study selection and data extraction for the systematic review. MSH/NA wrote the first draft of the manuscript. All authors contributed to the interpretation of data and critical revision of the manuscript, and approved the final manuscript. All authors confirm to the accuracy or integrity of the work.
Compliance with ethical standards
Conflicts of interest
The authors declare no conflict of interest directly applicable to this research.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rohit Saha and Julie Wilson are equal contributors
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third International consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. Recognizing sepsis as a global health priority—a WHO resolution. N Engl J Med. 2017;377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 3.Shankar-Hari M, Harrison DA, Rubenfeld GD, Rowan K. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br J Anaesth. 2017;119:626–636. doi: 10.1093/bja/aex234. [DOI] [PubMed] [Google Scholar]
- 4.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 5.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K, International Forum of Acute Care T Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 6.Shankar-Hari M, Harrison DA, Ferrando-Vivas P, Rubenfeld GD, Rowan K. Risk factors at index hospitalization associated with longer-term mortality in adult sepsis survivors. JAMA Netw Open. 2019;2:e194900. doi: 10.1001/jamanetworkopen.2019.4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA. 2018;319:62–75. doi: 10.1001/jama.2017.17687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shankar-Hari M, Rubenfeld GD. Understanding long-term outcomes following sepsis: implications and challenges. Curr Infect Dis Rep. 2016;18:37. doi: 10.1007/s11908-016-0544-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prescott HC, Langa KM, Iwashyna TJ. Readmission diagnoses after hospitalization for severe sepsis and other acute medical conditions. JAMA. 2015;313:1055–1057. doi: 10.1001/jama.2015.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar-Hari M, Ambler M, Mahalingasivam V, Jones A, Rowan K, Rubenfeld GD. Evidence for a causal link between sepsis and long-term mortality: a systematic review of epidemiologic studies. Crit Care. 2016;20:101. doi: 10.1186/s13054-016-1276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krumholz HM, Wang K, Lin Z, Dharmarajan K, Horwitz LI, Ross JS, Drye EE, Bernheim SM, Normand ST. Hospital-readmission risk—isolating hospital effects from patient effects. N Engl J Med. 2017;377:1055–1064. doi: 10.1056/NEJMsa1702321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnelly JP, Hohmann SF, Wang HE. Unplanned readmissions after hospitalization for severe sepsis at academic medical center-affiliated hospitals. Crit Care Med. 2015;43:1916–1927. doi: 10.1097/CCM.0000000000001147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prescott HC. Variation in post-sepsis readmission patterns: a cohort study of veterans affairs beneficiaries. Ann Am Thoracic Soc. 2016;14:230–237. doi: 10.1513/AnnalsATS.201605-398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002;359:248–252. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 15.Rothman KJ, Greenland S. Causation and causal inference in epidemiology. Am J Public Health. 2005;95(Suppl 1):S144–150. doi: 10.2105/AJPH.2004.059204. [DOI] [PubMed] [Google Scholar]
- 16.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861–870. doi: 10.1093/ije/dyr213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pivodic L, Pardon K, Morin L, Addington-Hall J, Miccinesi G, Cardenas-Turanzas M, Onwuteaka-Philipsen B, Naylor W, Ruiz Ramos M, Van den Block L, Wilson DM, Loucka M, Csikos A, Rhee YJ, Teno J, Deliens L, Houttekier D, Cohen J. Place of death in the population dying from diseases indicative of palliative care need: a cross-national population-level study in 14 countries. J Epidemiol Commun Health. 2016;70:17–24. doi: 10.1136/jech-2014-205365. [DOI] [PubMed] [Google Scholar]
- 19.Bekelman JE, Halpern SD, Blankart CR, Bynum JP, Cohen J, Fowler R, Kaasa S, Kwietniewski L, Melberg HO, Onwuteaka-Philipsen B, Oosterveld-Vlug M, Pring A, Schreyogg J, Ulrich CM, Verne J, Wunsch H, Emanuel EJ. Comparison of site of death, health care utilization, and hospital expenditures for patients dying with cancer in 7 developed countries. JAMA. 2016;315:272–283. doi: 10.1001/jama.2015.18603. [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Bone RC, Sprung CL, Sibbald WJ. Definitions for sepsis and organ failure. Crit Care Med. 1992;20:724–726. doi: 10.1097/00003246-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, Sccm/Esicm/Accp/Ats/Sis 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 24.Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schunemann H, Guyatt G. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015;350:h870. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 25.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun L, Riedel AA, Cooper LM. Severe sepsis in managed care: analysis of incidence, 1-year mortality, and associated costs of care. J Manag Care Pharm. 2004;10:521–530. doi: 10.18553/jmcp.2004.10.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cakir B, Gammon G. Evaluating readmission rates: how can we improve? South Med J. 2010;103:1079–1083. doi: 10.1097/SMJ.0b013e3181f20a0f. [DOI] [PubMed] [Google Scholar]
- 29.Chang DW, Tseng CH, Shapiro MF. Rehospitalizations following sepsis: common and costly. Crit Care Med. 2015;43:2085–2093. doi: 10.1097/CCM.0000000000001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deb P, Murtaugh CM, Bowles KH, Mikkelsen ME, Khajavi HN, Moore S, Barron Y, Feldman PH. Does early follow-up improve the outcomes of sepsis survivors discharged to home health care? Med Care. 2019;57:633–640. doi: 10.1097/MLR.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 31.DeMerle KM, Vincent BM, Iwashyna TJ, Prescott HC. Increased healthcare facility use in veterans surviving sepsis hospitalization. J Crit Care. 2017;42:59–64. doi: 10.1016/j.jcrc.2017.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DeMerle KM, Royer SC, Mikkelsen ME, Prescott HC. Readmissions for recurrent sepsis: new or relapsed infection? Crit Care Med. 2017;45:1702–1708. doi: 10.1097/CCM.0000000000002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dick A, Liu H, Zwanziger J, Perencevich E, Furuya EY, Larson E, Pogorzelska-Maziarz M, Stone PW. Long-term survival and healthcare utilization outcomes attributable to sepsis and pneumonia. BMC Health Serv Res. 2012;12:432. doi: 10.1186/1472-6963-12-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietz BW, Jones TK, Small DS, Gaieski DF, Mikkelsen ME. The relationship between index hospitalizations, sepsis, and death or transition to hospice care during 30-day hospital readmissions. Med care. 2016;55:362–370. doi: 10.1097/MLR.0000000000000669. [DOI] [PubMed] [Google Scholar]
- 35.Gadre SK, Shah M, Mireles-Cabodevila E, Patel B, Duggal A. Epidemiology and predictors of 30-day readmission in patients with sepsis. Chest. 2019;155:483–490. doi: 10.1016/j.chest.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin AJ, Rice DA, Simpson KN, Ford DW. Frequency, cost, and risk factors of readmissions among severe sepsis survivors. Crit Care Med. 2015;43:738–746. doi: 10.1097/CCM.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guirgis FW, Brakenridge S, Sutchu S, Khadpe JD, Robinson T, Westenbarger R, Topp ST, Kalynych CJ, Reynolds J, Dodani S, Moore FA, Jones AE. The long-term burden of severe sepsis and septic shock: sepsis recidivism and organ dysfunction. J Trauma Acute Care Surg. 2016;81:525–532. doi: 10.1097/TA.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 38.Hua M, Gong MN, Brady J, Wunsch H. Early and late unplanned rehospitalizations for survivors of critical illness. Crit Care Med. 2015;43:430–438. doi: 10.1097/CCM.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones TK, Fuchs BD, Small DS, Halpern SD, Hanish A, Umscheid CA, Baillie CA, Kerlin MP, Gaieski DF, Mikkelsen ME. Post-acute care use and hospital readmission after sepsis. Ann Am Thoracic Soc. 2015;12:904–913. doi: 10.1513/AnnalsATS.201411-504OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JS, Kim YJ, Ryoo SM, Sohn CH, Ahn S, Seo DW, Lim KS, Kim WY. Risk factors for same pathogen sepsis readmission following hospitalization for septic shock. J Clin Med. 2019;8:181. doi: 10.3390/jcm8020181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu V, Lei X, Prescott HC, Kipnis P, Iwashyna TJ, Escobar GJ. Hospital readmission and healthcare utilization following sepsis in community settings. J Hosp Med. 2014;9:502–507. doi: 10.1002/jhm.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayr FB, Talisa VB, Balakumar V, Chang CH, Fine M, Yende S. Proportion and cost of unplanned 30-day readmissions after sepsis compared with other medical conditions. JAMA. 2017;317:530–531. doi: 10.1001/jama.2016.20468. [DOI] [PubMed] [Google Scholar]
- 43.Meyer N, Harhay MO, Small DS, Prescott HC, Bowles KH, Gaieski DF, Mikkelsen ME. Temporal trends in incidence, sepsis-related mortality, and hospital-based acute care after sepsis. Crit Care Med. 2018;46:354–360. doi: 10.1097/CCM.0000000000002872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nkemdirim Okere A, Renier CM. Effects of statins on hospital length of stay and all-cause readmissions among hospitalized patients with a primary diagnosis of sepsis. Ann Pharmacother. 2015;49:1273–1283. doi: 10.1177/1060028015603072. [DOI] [PubMed] [Google Scholar]
- 45.Norman BC, Cooke CR, Ely EW, Graves JA. Sepsis-associated 30-day risk-standardized readmissions: analysis of a nationwide medicare sample. Crit Care Med. 2017;45:1130–1137. doi: 10.1097/CCM.0000000000002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nsutebu EF, Ibarz-Pavon AB, Kanwar E, Prospero N, French N, McGrath C. Advancing quality in sepsis management: a large-scale programme for improving sepsis recognition and management in the North West region of England. Postgrad Med J. 2018;94:463–468. doi: 10.1136/postgradmedj-2018-135833. [DOI] [PubMed] [Google Scholar]
- 47.Ortego A, Gaieski DF, Fuchs BD, Jones T, Halpern SD, Small DS, Sante SC, Drumheller B, Christie JD, Mikkelsen ME. Hospital-based acute care use in survivors of septic shock. Crit Care Med. 2015;43:729–737. doi: 10.1097/CCM.0000000000000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prescott HC, Dickson RP, Rogers MA, Langa KM, Iwashyna TJ. Hospitalization type and subsequent severe sepsis. Am J Respir Crit Care Med. 2015;192:581–588. doi: 10.1164/rccm.201503-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prescott HC, Langa KM, Liu V, Escobar GJ, Iwashyna TJ. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014;190:62–69. doi: 10.1164/rccm.201403-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schnegelsberg A, Mackenhauer J, Nibro HL, Dreyer P, Koch K, Kirkegaard H. Impact of socioeconomic status on mortality and unplanned readmission in septic intensive care unit patients. Acta Anaesthesiol Scand. 2016;60:465–475. doi: 10.1111/aas.12644. [DOI] [PubMed] [Google Scholar]
- 51.Singh A, Bhagat M, George SV, Gorthi R, Chaturvedula C. Factors associated with 30-day unplanned readmissions of sepsis patients: a retrospective analysis of patients admitted with sepsis at a community hospital. Cureus. 2019;11:e5118. doi: 10.7759/cureus.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun A, Netzer G, Small DS, Hanish A, Fuchs BD, Gaieski DF, Mikkelsen ME. Association between index hospitalization and hospital readmission in sepsis survivors. Crit Care Med. 2016;44:478–487. doi: 10.1097/CCM.0000000000001464. [DOI] [PubMed] [Google Scholar]
- 53.Sutton JP, Friedman B (2013) Trends in septicemia hospitalizations and readmissions in selected HCUP States, 2005 and 2010: statistical brief #161Healthcare Cost and utilization project (HCUP) statistical briefs. Agency for Healthcare Research and Quality (US), Rockville (MD) [PubMed]
- 54.Vashi AA, Fox JP, Carr BG, D'Onofrio G, Pines JM, Ross JS, Gross CP. Use of hospital-based acute care among patients recently discharged from the hospital. JAMA. 2013;309:364–371. doi: 10.1001/jama.2012.216219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang T, Derhovanessian A, De Cruz S, Belperio JA, Deng JC, Hoo GS. Subsequent infections in survivors of sepsis: epidemiology and outcomes. J Intensive Care Med. 2014;29:87–95. doi: 10.1177/0885066612467162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weinreich MA, Styrvoky K, Chang S, Girod CE, Ruggiero R. Sepsis at a safety net hospital: risk factors associated with 30-day readmission. J Intensive Care Med. 2017 doi: 10.1177/0885066617726753. [DOI] [PubMed] [Google Scholar]
- 57.Wong EL, Cheung AW, Leung MC, Yam CH, Chan FW, Wong FY, Yeoh EK. Unplanned readmission rates, length of hospital stay, mortality, and medical costs of ten common medical conditions: a retrospective analysis of Hong Kong hospital data. BMC Health Serv Res. 2011;11:149. doi: 10.1186/1472-6963-11-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yende S, Kellum JA, Talisa VB, Peck Palmer OM, Chang CH, Filbin MR, Shapiro NI, Hou PC, Venkat A, LoVecchio F, Hawkins K, Crouser ED, Newman AB, Angus DC. Long-term host immune response trajectories among hospitalized patients with sepsis. JAMA Netw Open. 2019;2:e198686. doi: 10.1001/jamanetworkopen.2019.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zilberberg MD, Shorr AF, Micek ST, Kollef MH. Risk factors for 30-day readmission among patients with culture-positive severe sepsis and septic shock: a retrospective cohort study. J Hosp Med. 2015;10:678–685. doi: 10.1002/jhm.2420. [DOI] [PubMed] [Google Scholar]
- 60.Ahmad S, Baig S, Taneja A, Nanchal R, Kumar G. The outcomes of severe sepsis in homeless. Chest. 2014;146:230. [Google Scholar]
- 61.Bath AS, Farishta M, Deepak V. Trends and 30-day readmission rate for patients discharged with septicemia: analysis of 3,082,888 admissions. Am J Respir Crit Care Med. 2019;199:3457. [Google Scholar]
- 62.Demiralp B, He F, Koenig L, Hengesbach D, Prister J. A regional analysis of patient outcomes and medicare payments for sepsis patients treated in long-term care hospitals and skilled nursing facilities. Am J Respir Crit Care Med. 2017;195:5023. [Google Scholar]
- 63.Galiatsatos P, Follin A, Uradu N, Alghanim F, Daniel Y, Saria S, Townsend J, Sylvester C, Chanmugam A, Chen ES. The association between neighborhood socioeconomic disadvantage and readmissions for patients hospitalized with sepsis. Am J Respir Crit Care Med. 2019;199:5569. doi: 10.1097/CCM.0000000000004307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jones TK, Fuchs BD, Small DS, Halpern SD, Hanish A, Baillie C, Umscheid CA, Kerlin MP, Gaieski DF, Mikkelsen ME. Severe sepsis is associated with high rates of hospital readmission. Am J Respir Crit Care Med. 2014;189:79–83. [Google Scholar]
- 65.Kaplan M, Chowdhury MAB, Hurwitz J, Metellus V, Martin E, Elie MC. Sepsis recidivism: return visits and recurrence (S3R analysis) Ann Emerg Med. 2017;70:S60–S60. [Google Scholar]
- 66.Kopterides P, Diane I, Lucko N, Shapiro N, Hou P, Filbin M, Kellum J, Yende S. Rehospitalizations and fatal outcome are common among severe sepsis survivors. Crit Care Med. 2015;43:252. [Google Scholar]
- 67.Leu M, Gonzalez C, Rico-Crescencio J, Kaur T, Kanna B, Loganathan R. Survivors of severe sepsis/septic shock—a need to focus on healthcare utilization post-discharge among inner-city minority New-Yorkers. Am J Respir Crit Care Med. 2013;187:502–507. [Google Scholar]
- 68.Leung S, Gong MN. Healthcare utilization prior to and following patients admitted to the ICU with severe sepsis. Am J Respir Crit Care Med. 2012;185:513–516. [Google Scholar]
- 69.Mayr F, Balakumar V, Talisa V, Fine M, Yende S. Understanding the burden of unplanned sepsis readmissions. Crit Care Med. 2016;44:409. [Google Scholar]
- 70.Perakis PG, Rubinfeld I, Narayana A, Cumba M, Takis L, Coba V, Blyden D, Horst M. Hospital readmission incidence for septic patients from the surgical intensive care unit. Shock. 2012;37:76–76. [Google Scholar]
- 71.Prescott HC, Langa KM, Iwashyna TJ. Forty percent of hospitalizations after severe sepsis are potentially preventable. Am J Respir Crit Care Med. 2015;191:35. [Google Scholar]
- 72.Rice D, Goodwin AJ, Simpson K, Ford DW. Risk factors for 30-day hospital readmissions in sepsis. Am J Respir Crit Care Med. 2014;189:322–330. [Google Scholar]
- 73.Rice D, Simpson K, Goodwin A, Ford D. Patterns of hospital readmission following an index hospitalization for sepsis. Chest. 2013;144:400. [Google Scholar]
- 74.Rico Crescencio JC, Leu M, Balaventakesh B, Loganathan R. REadmissions among patients with severe sepsis/septic shock among inner-city minority new yorkers. Chest. 2012;142:286A–286A. [Google Scholar]
- 75.Rudym D, Uppal A, Evans L. Readmission rates in severe sepsis. Am J Respir Crit Care Med. 2015;191:2085–2093. [Google Scholar]
- 76.Styrvoky K, Weinreich M, Girod C, Ruggiero R. Beyond surviving sepsis: thirty-day readmission rates and patient characteristics at a safety net hospital. Chest. 2015;148:336. [Google Scholar]
- 77.Styrvoky K, Weinreich M, Girod CE, Ruggiero RM. Risk factors for thirty-day readmissions among sepsis survivors at a safety net hospital. Am J Respir Crit Care Med. 2016;193:3676. [Google Scholar]
- 78.Tsen R, Seoane L, Li D, Pavlov A, Winterbottom F, Nash T. Frequency and timing of readmissions among survivors of severe sepsis and septic shock. Am J Respir Crit Care Med. 2015;191:6240. [Google Scholar]
- 79.Whiles B, Deis A, Miller P, Simpson S. Comorbid conditions predict outcomes in patients with severe sepsis. Chest. 2016;149:A170. doi: 10.1016/j.chest.2016.02.194. [DOI] [PubMed] [Google Scholar]
- 80.Nussbaumer-Streit B, Klerings I, Dobrescu AI, Persad E, Stevens A, Garritty C, Kamel C, Affengruber L, King VJ, Gartlehner G. Excluding non-english publications from evidence-syntheses did not change conclusions: a meta-epidemiological study. J Clin Epidemiol. 2019;118:42–54. doi: 10.1016/j.jclinepi.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 81.Morrison A, Polisena J, Husereau D, Moulton K, Clark M, Fiander M, Mierzwinski-Urban M, Clifford T, Hutton B, Rabb D. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care. 2012;28:138–144. doi: 10.1017/S0266462312000086. [DOI] [PubMed] [Google Scholar]
- 82.Dharmarajan K, Wang Y, Lin Z, Normand ST, Ross JS, Horwitz LI, Desai NR, Suter LG, Drye EE, Bernheim SM, Krumholz HM. Association of changing hospital readmission rates with mortality rates after hospital discharge. JAMA. 2017;318:270–278. doi: 10.1001/jama.2017.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prescott HC, Sjoding MW, Iwashyna TJ. Diagnoses of early and late readmissions after hospitalization for pneumonia. A systematic review. Ann Am Thorac Soc. 2014;11:1091–1100. doi: 10.1513/AnnalsATS.201404-142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Riley RD, Moons KGM, Snell KIE, Ensor J, Hooft L, Altman DG, Hayden J, Collins GS, Debray TPA. A guide to systematic review and meta-analysis of prognostic factor studies. BMJ. 2019;364:k4597. doi: 10.1136/bmj.k4597. [DOI] [PubMed] [Google Scholar]
- 85.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M, Sepsis Definitions Task F. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riche F, Chousterman BG, Valleur P, Mebazaa A, Launay JM, Gayat E. Protracted immune disorders at one year after ICU discharge in patients with septic shock. Crit Care. 2018;22:42. doi: 10.1186/s13054-017-1934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shankar-Hari M, Harrison DA, Rowan KM, Rubenfeld GD. Estimating attributable fraction of mortality from sepsis to inform clinical trials. J Crit Care. 2018;45:33–39. doi: 10.1016/j.jcrc.2018.01.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.