Table 5.
Selenium containing compounds for cancer treatment
| S.no | Name of the compounds | Structure | Types of cancer/cell lines | Dosage concentration in micromolar (µM) | Apoptosis mechanism | References |
|---|---|---|---|---|---|---|
| Inorganic selenium containing compounds | ||||||
| 1 | Sodiu m selenite | 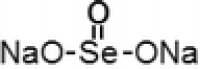 |
1) HSC-3, HSC-4, and SAS | 5–100 µM for 2–5 days | Pronounced anti-proliferative effect of selenite against three oral cancer cell lines | Endo et al. (2017) |
| 2) Lung cancer cell lines (H157, H611, and U2020) | 5 µM for 5 h | Played a role in natural killer (NK) cell-based anticancer immunotherapy where it could increase the susceptibility of cancer cells to CD94/NK group 2A-positive NK cells | Olm et al. (2009) | |||
| 3) HepG2, HeLa, and MCF-7 cells | – | Apoptosis of cancer cells occurs by accumulation in mitochondria which would subsequently damage mitochondrial function, structure and lead to cell death | Hu et al. (2018) | |||
| 2 | Sodium selenate | 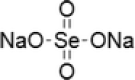 |
Human oral squamous carcinoma (KB) cells resistant to chemotherapeutic drug vincristine (KBV20C) | 5, 10, 30, and 50 µM for 48 h | Produced a higher sensitizing effect on the KBV20C cells by arresting the cell cycle at G2-phase and activating apoptotic pathways | Choi et al. (2015) |
| Natural organoselenium compounds | ||||||
| 3 | Selenomethionine | 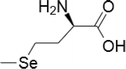 |
1) Lung cancer cells -NCI-H460 and H1299 | 10–1000 μM | SeMet used in combination with ionizing radiation enhanced treatment | Yang et al. (2009) |
| 2) Mouse xenograft model of colorectal carcinoma (SW480) | 10–1000 μM | Low systemic toxicity and high tumor selectivity has been reported | Shin et al. (2007) | |||
| 3) Colon cancer | 100–1000 μM | Participation of decreased COX-2 expression | Baines et al. (2002) | |||
| 4) Breast and prostate cancer | 10–1000 μM | Cell death is facilitated by caspases and ER stress | Suzuki et al. (2010a) | |||
| 4 | Selenocystine | 1) Cervical cancer | 100–1000 μM | Paraptotic-like mediated by ER stress and UPR | Wallenberg et al. (2014) | |
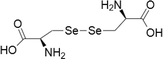 |
2) Nasopharyngeal, Liver, Lung and Melanoma | 1–20 μM | Cell death is facilitated by caspases, mitochondrial dysfunction/signalling and PARP cleavage | Poerschke and Moos (2011) | ||
| 3) Acute promyelocytic leukemia, | 10–100 μM | |||||
| 4) Colon cancer | 1–100 μM | |||||
| 5 | Methylseleno-cysteine | 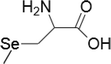 |
Breast cancer | 100–1000 μM | Cell death is facilitated by caspases, ER stress, mitochondrial dysfunction/signalling and PARP cleavage | Suzuki et al. (2010b) |
| Colon cancer | 10–1000 μM | |||||
| Lung cancer | 10–1000 μM | |||||
| Oral Squamous | 10–1000 μM | |||||
| 6 | Selenodiglutathione | 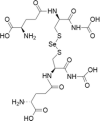 |
Acute myeloid leukaemia | 1–20 μM | Cell death is facilitated by ROS production and oxidative damage | Wallenberg et al. (2014); Tobe et al. (2015) |
| Breast cancer | 1–20 μM | |||||
| Cervical cancer | 1–20 μM | |||||
| Lymphoma | 1–20 μM | |||||
| Oral cancer | 0.1–2 μM | |||||
| Synthetic organoselenium compounds | ||||||
| 7 | Methylseleninic acid | Lung cancer | 10–100 μM | Cell death is facilitated by caspases, ER stress, UPR, mitochondrial dysfunction/signalling and PARP cleavage | Poerschke and Moos (2011);Wang et al. (2014); Shigemi et al. (2017) | |
| Pancreatic cancer | 0.1–2 μM | |||||
| Lymphoma | 1–100 μM | |||||
| 8 | Ebselen | 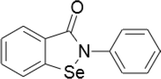 |
Bone marrow cancer | 10–100 μM | Cell death is mediated by mitochondrial signaling. Acts as a substrate for thioredoxin reductase and rapidly oxidizes thioredoxin, leading to oxidative stress | Zhang et al. (2014) |
| myeloma | ||||||
| 9 | Ethaselen | 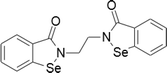 |
Tongue cancer | 1–20 μM | Cell death is mediated by thioredoxin reductase inhibition and subsequent oxidative stress | Xing et al. (2008); Wang et al. (2012) |
| cervival, gastric, liver, lung cancers | 0.1–2 μM | |||||
| 10 | Diselenides | 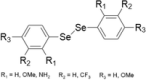 |
Colon cancer cells | 1–100 μM | Cytotoxicity by cell-cycle arrest and caspase pathway | Nedel et al. (2012) |
| 11 | Selenazofurin | 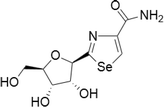 |
MCF-7 human breast carcinoma cells | 0.1–2 μM | Pro-apoptotic activity and higher growth inhibition | Zhou et al. (2015) |
| Bladder, cervical, colon, leukemia, lymphoma, kidney | 0.1–2 μM in blood cell tumours, 1–20 μM in solid tumours | Act through non-competitive inhibition of inosine monophosphate dehydrogenase, thus limiting de novo guanine nucleotide biosynthesis | Franchetti et al. (1997) | |||
| 12 | Selenocyantes | 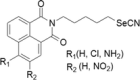 |
CCRF-CEM, HT-29, HTB-54, PC-3 and, MCF-7 cell lines | 1–100 μM |
Increase in compound lipophilicity and thereby increasing the alkyl chain length was consistent In cells associated to a caspase-dependent apoptotic cell death through the induction of p53, Bax and suppression of Bcl-2 |
Romano et al. (2015) |
| 13 | Selenoesters | 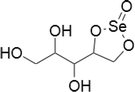 |
Prostate, breast, lung and colon cancer cell lines | Below 0.1 μM | Acting as GSH depleting agents | Dominguez-Alvarez et al. (2014) |
| Human hepatoma cells | Ability to act as strong inhibitor of cellular efflux pump P-gp | |||||
| In mouse MDR T-lymphoma cells and in human colon cancer cells | ||||||
| 14 | Se-NSAID | 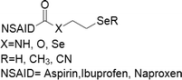 |
Breast, colorectal, melanoma and pancreatic cancers | 1–100 μM | Apoptosis mediated by caspases, PARP cleavage and ROS production. Involvement of COX-2 and PI3K/AKT inhibition | Gowda et al. (2013); Plano et al. (2016) |
| 15 | Selenoureas | 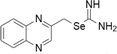 |
Colon cancer cell models | Below 10 μM | Activation of caspase-dependent pathways and inhibition of antiapoptotic proteins | Alcolea et al. (2016) |
| 16 | Selenocarbonyl derivatives | 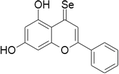 |
MCF-7 cells | 1–100 μM | Arresting the cell cycle and caspase pathway | Martins et al. (2015) |
Dosage concentration (in vitro dose for 48–72 h IC50)—very low (0.1–2 μM), low (1–20 μM), low to medium (1–100 μM), medium (10–100 μM), medium to high (10–1000 μM, high (100–1000 μM)
