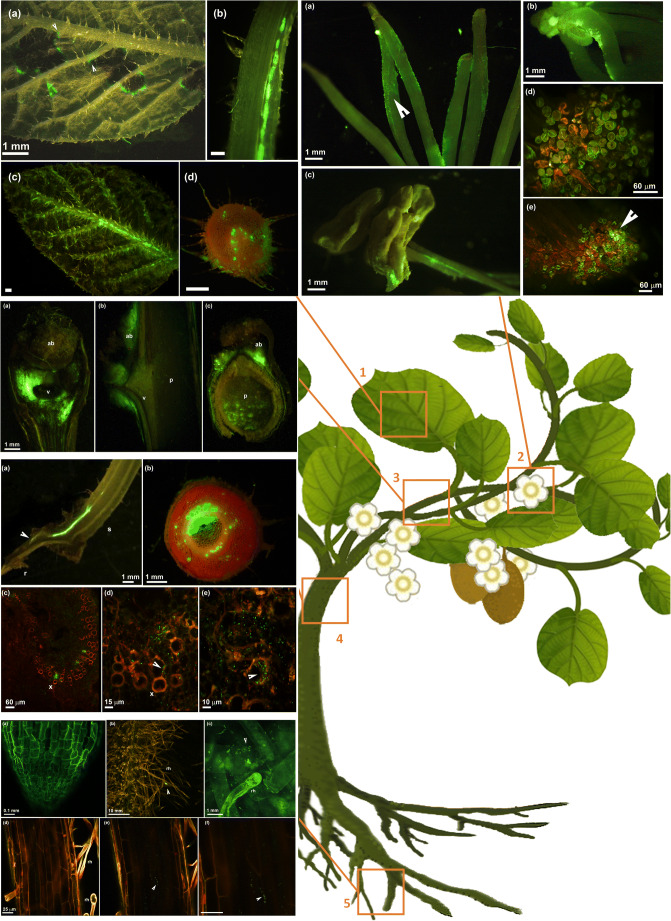
Fig. 1.
Different organs of kiwifruit plant colonized by Pseudomonas syringae pv. actinidiae expressing a green fluorescent protein (CFBP7286-GFPuv): (1) leaf infection, (2) flower infection, (3) infection of leaf abscission scar and colonization of developed bud, (4) migration inside xylem vessel, (5) colonization of roots. 1. Fluorescence stereomicroscope photographs of leaf infection in A. chinensis var. deliciosa (a-b-d) and A. chinensis var. chinensis(c). a Leaf showing angular necrotic spots. The necrotic spots are surrounded by a bright green edge caused by GFPuv-labelled Psa, indicating that the pathogen successfully evaded the necrotic area and the possible programmed cell death enacted by the host to contain the pathogen spread. The angular spots can cover leaf lateral veins, suggesting the possible entry of the pathogen into the vascular system. b Migration of Psa inside the leaf petiole, leading to a systemic infection of the host plant. cA. chinensis var. chinensis young leaf infected by GFPuv-labelled Psa. Even in absence of leaf symptoms, the central leaf vein was colonized. d Transversal section of a leaf petiole showing the xylem vessels fully colonized by GFPuv-labelled Psa. The cortical parenchyma and the epidermis were also colonized, suggesting that Psa migration from the leaves to the shoots may occur both inside the petiole and along it by epiphytic growth. In all panels, the measure bar is 1 mm. 2. Flower infection in A. chinensis var. deliciosa (a, b) and A. chinensis var. chinensis (c, d, e). a Fluorescence stereomicroscope photograph of stigmas and styles colonized by GFPuv-labelled Psa. The pathogen migrates epiphytically along the style by forming a biofilm covering the surface of the style (arrow). b Fluorescence stereomicroscope photograph showing the epiphtytic colonization of stigmas inoculated by pollen contaminated with by GFPuv-labelled Psa. c Fluorescence stereomicroscope photograph of dehiscent anthers colonized by GFPuv-labelled Psa. The pathogen colonized also the pedicels. d, e Confocal laser scanning micrograph showing stigmas coated by pollen grains contaminated with by GFPuv-labelled Psa. The arrow indicates an area were a successful colonization of the stigma by the pathogen occurred. The measure bar is indicated in each panel. 3. Fluorescence stereomicroscope photograph of A. chinensis var. deliciosa leaf abscission scars infected by GFPuv-labelled Psa. a photograph of the abscission scar taken from above. The whole area corresponding to the abscission scar is characterized by a bright green signal due to pathogen colonization; ab: axillary bud, v: vascular bundle corresponding the junction between the leaf petiole and the lignified shoot. b Longitudinal and c transversal sections of the leaf scar. The tissues surrounding the scar show a bright green signal. The axillary bud is also colonized; p: pith. In all panels, the measure bar is 1 mm. 4. Systemic colonization of Actinidia plants via migration inside the xylem vessels. a Fluorescence stereomicroscope photograph of a longitudinal section of a young A. chinensis var. deliciosa seedling approximately 5 cm tall. Inoculation was performed on the shoot tip. Psa colonization is concentrated in the vascular bundle inside the main shoot (s) up to the taproot (r). b Fluorescence stereomicroscope photograph of a transversal section of the main stem of a young A. chinensis var. deliciosa seedling. Psa heavily colonized the primary and secondary xylem vessels and pith. Sporadic presence of Psa is also visible in sieve elements and sclerenchyma fibres in the cortex. c–d confocal laser scanning micrographs showing xylem vessels (x) colonized by Psa. Each green rod corresponds to a pathogen cell. The arrow indicates Psa cell attached to the internal wall of the xylem vessels. The measure bar is indicated in each panel. 5. Confocal laser scanning micrograph of Actinidia roots. a Uninfected root tip. b Root hairs. The arrow indicates a Psa microcolony growing on the root surface. c Magnification of root hairs colonized by Psa. Rh: root hair. Each green rod corresponds to a pathogen cell. d-e-f Micrograph of a fine root surrounded by root hairs. d External surface of the root without the presence of Psa; e internal tissues inside the root where Psa microcolonies are visible; f magnification of Psa microcolonies inside the root. In all panels, the measure bar is 25 μm