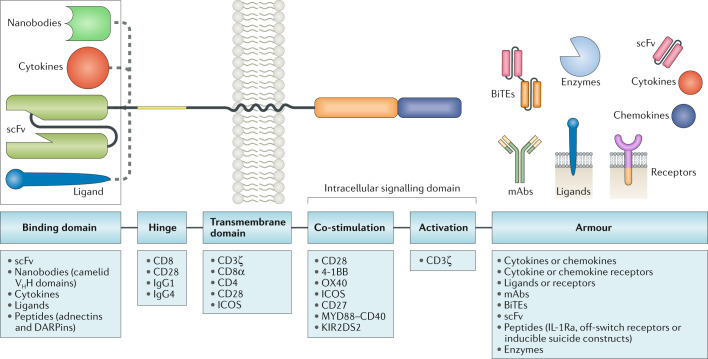
Fig. 1. Blueprint of CAR design.
Chimeric antigen receptors (CARs) have a modular design consisting of an antigen-binding domain, a hinge, a transmembrane domain and an intracellular signalling domain. In preclinical and clinical studies of CAR T cells, investigators have used reference sequences from a myriad of molecules within each of these domains. The antigen-binding domain is usually a single-chain variable fragment (scFv) molecule derived from a monoclonal antibody (mAb; from mouse anti-human CD19 antibodies, for example, in the currently FDA-approved CAR T cell products). The intracellular signalling domain generally contains a T cell activation domain derived from the CD3ζ chain of the T cell receptor as well as co-stimulatory domains that often comprise immunoreceptor tyrosine-based activation motif-containing regions of CD28 or 4-1BB (also known as CD137 and TNFRSF9). CAR gene constructs can be further modified to engineer CAR T cells with expression of an ‘armour’ protein, which is typically a cell-surface or secreted immunomodulatory molecule that enhances T cell function or favourably modifies the tumour microenvironment. Variation of each of these component parts of CAR constructs enables fine tuning of the functionality and antitumour activity of the resultant CAR T cell product, and CARs with various designs are being developed to improve the safety and efficacy of these therapies across various cancers. In addition, gene editing of the engineered T cells to further enhance CAR T cell function is a promising avenue of research in this area. BiTEs, bi-specific T cell engagers; DARPins, designed ankyrin repeat proteins; ICOS, inducible T cell co-stimulator; IL-1Ra, IL-1 receptor antagonist; KIR2DS2, killer cell immunoglobulin-like receptor 2DS2; VHH, variable domain of a heavy chain antibody.