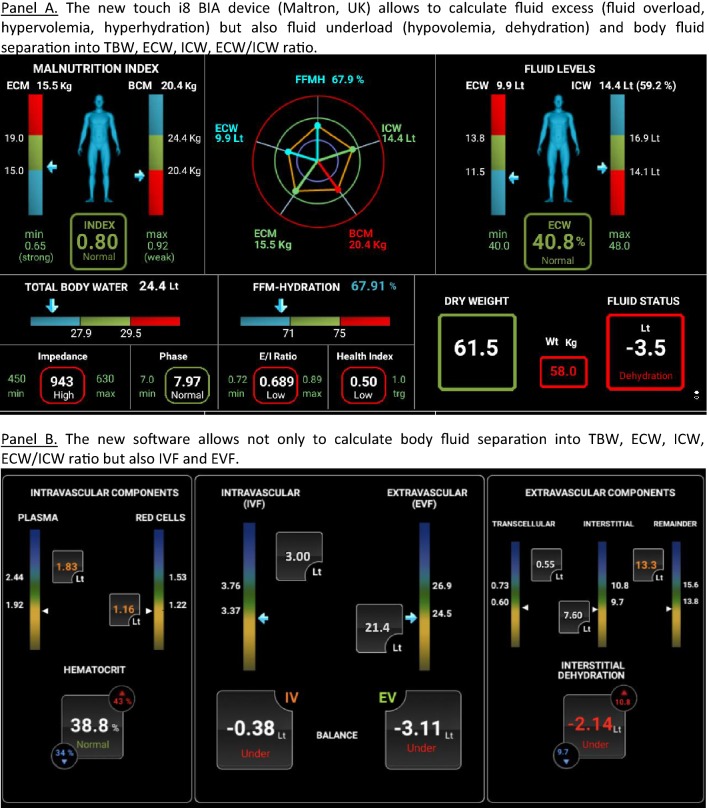
The burden of fluid overload
Fluid overload in critically ill patients is defined as a 10% increase in cumulative fluid balance (CFB) from baseline body weight and represents a well-known problem which detrimentally affects intensive care unit (ICU) patient’s clinical course and their outcome [1–3]. Therefore, timely recognition and correction of fluid overload, or even better, identification of patients at risk for fluid accumulation in an early stage is of great importance. However, this remains challenging in clinical practice, as we miss an accurate and reliable tool for correct assessment of fluid status [4]. Current methods include the calculation of daily and CFB, by recording fluid inputs and outputs, the use of clinical or biochemical signs of fluid overload, monitoring filling pressures, however, none of these methods allow for a close monitoring for fluid balance, and intercompartmental fluid shifts [1, 5].
The promise for BIA
Bio-electrical impedance analysis (BIA) has gained increased interest to help physicians to determine volume status and fluid distribution in critically ill patients (see Table 1) [1, 2, 4, 6–21]. Indeed, several data published in the last decade suggest that BIA may provide useful information not only in different well-established patient groups (dialysis, AIDS, malnutrition), but also in critically ill patients with burns, trauma or sepsis undergoing fluid resuscitation. This tool may offer a non-invasive, fast and reliable assessment of volume status and fluid distribution as well as evaluation of dynamic changes in fluid distribution. It measures total body water (TBW), extracellular water (ECW), and intracellular water (ICW). BIA can calculate absolute fluid overload (AFO), the difference between normal, expected ECW and the actual, measured ECW, expressed in liters, as well as relative fluid overload (RFO), AFO/ECW, expressed in percentages. BIA measurements may be performed easily at the bedside, do not require extensive training and have limited inter-observer variations [22]. Thereby, BIA sounds as a promising diagnostic tool in the ICU or operating room to assess fluid status. Recently, new BIA-devices have been introduced allowing not only calculation of TBW, ECW and ICW but also the intra- and extravascular fluids (IVF, EVF) and, in case of dialysis, Kt/V (Fig. 1).
Table 1.
Overview of recent studies published on the use of bio-electrical impedance analysis in critically ill patients
| Author | Year | Type of study | Population | Number of patients | Protocol | Definition FO | Most important outcome |
|---|---|---|---|---|---|---|---|
| Bracco et al. [7] | 1998 | Prospective observational study | Adult patients undergoing open-heart surgery with cardiopulmonary bypass | 26 | To assess fluid accumulation after cardiac surgery by multiple frequency segmental BIA | No definition | Cardiac surgery produced a significant decrease in segmental trunk BIA, reflecting fluid accumulation at the trunk level |
| Piccoli et al. [15] | 2000 | Propective cross-sectional study | 72% surgical patients, 28% craniocerebral trauma and bleeding | 121 | To determine relationships between CVP and tissue electrical conductivity measurements according to the BIVA, with also TBW predictions based both on anthropometry and conventional BIA regression equations | Short vectors out of the lower pole of the target 75% tolerance ellipse | (1) The agreement between BIVA and CVP indications was good in the high CVP group, moderate in the medium CVP group, and poor in low CVP group. (2) Direct impedance measurements were more highly correlated with CVP values than with TBW predictions from either conventional BIA equations or anthropometry |
| Lingwood et al. [20] | 2000 | Prospective study | NICU patients | 24 | To investigate the effects of factors like cardio-respiratory monitoring equipment, non-ideal electrode placement and inability to obtain accurate crown-heel measurements on impedance analysis and to develop a prediction equation for ECF volume in the neonate | NA | Despite many potential difficulties associated with impedance analysis in the NICU, reliable measurements of impedance can be obtained |
| House et al. [18] | 2010 | Prospective study | Adult ICU patients on mechanical ventilation | 34 | Relationships were assessed between CVP, BIVA, BNP, and oxygenation index (O2I) in a cross-sectional (baseline) and longitudinal fashion using both univariate and multivariable modeling | NA | No addition was found in BIVA measures to help in the fluid assessment of ICU patients |
| Basso et al. [1] | 2013 | Prospective observational study | Mixed ICU patients: post-surgery, sepsis, trauma, post-cardiac arrest | 64 | To investigate: (i) the hydration status of ICU patients, and how this varies during hospitalization following current fluid management practices; (ii) the hydration status of ICU patients in need of RRT, and how this varies during treatment, and (iii) the relationship between hydration and mortality with or without RRT | Body hydration percentage > 73.3% | (1) A marked tendency towards overhydration during the 1st day in ICU. (2) Significantly worse state of hyperhydration in patients on CRRT. (3) Non-survivors showed worse hyperhydration patterns in comparison to survivors. (4) Both the average and the maximum hydration represent a significant risk factor for mortality, both in the ICU and at 60 days |
| Dabrowski et al. [9] | 2014 | Prospective study | Septic shock complicated with acute kidney injury | 30 | Analysis of the effect and the time course of CVVH with UF on IAP and body fluid volumes in septic shock patients with AKI | No definition. Comparison of various fluid volumes between survivors and non-survivors | IAP correlated with fluid volume excess measured by BIA |
| Ismael et al. [16] | 2014 | Prospective experimental study | Hemodynamically stable patients requiring acute RRT for fluid overload | 31 | To quantify BCM, water compartments and FFM by methods usable at the bedside for evaluating the impact of sudden and massive fluid shifts on body composition in ICU patients | Ultrafiltration estimated ≥ 5% body weight before the hemodialysis session | BCM estimation is less driven by sudden massive fluid shifts than FMM. Assessment of BCM should be preferred to FFM when severe hydration disturbances are present in ICU patients |
| Jones et al. [8] | 2015 |
Prospective, clinician-blinded, observational study |
Mechanically ventilated patients in mixed ICU | 61 | To assess the feasibility and validity of BIVA as a measure of hydration in critically ill patients | NA | BIVA is feasible in critically ill patients. Directional changes in BIVA were consistent with directional changes in fluid balance. The sensitivity of repeated BIVA hydration measurements to detect fluid accumulation or fluid balance changes < 2 L was low |
| Chen et al. [10] | 2015 | Prospective observational study | Mixed (medical-surgical) ICU patients | 89 | To assess fluid status among patients on CRRT by using BIVA combined with serum NT-pro BNP and to identify correlations between outcome and fluid status, as determined by the combination of these two parameters | Point vector on the RXc point graph falling lower than the lower pole of the 75% tolerance ellipse | Different types of fluid status distinguished by BIVA combined with serum NT-pro BNP measurements corresponded to different clinical conditions and treatment outcomes. For patients receiving CRRT, real-time monitoring of fluid status by using BIVA and NT-pro BNP may be useful in fluid management by aiding in the identification of an optimal net ultrafiltration rate during CRRT |
| Rhee et al. [17] | 2015 | Retrospective study | Male ICU patients with AKI on CVVHDF | 208 | To analyze the effect of MF–BIA-defined volume status on the mortality of critically ill patients with AKI | No definition | MF–BIA-defined excess TBW/H2 and ICW/H2 are independently associated with higher in-hospital mortality in male patients with AKI undergoing CVVHDF |
| Mól and Kwinta [19] | 2015 | Prospective study | Extreme premature and full-term newborns | 38 | Evaluation of BIA values and body composition during early infancy in groups of preterm newborns and full term newborns | NA | The study confirms differences in body composition between preterm and full term newborns |
| Dewitte et al. [4] | 2016 | Prospective observational study | Mechanically ventilated patients in mixed ICU | 25 | To evaluate the feasibility and reproducibility of BIS to measure body-water composition in critically ill patients, and to compare fluid balance and daily changes in TBW measured by bioimpedance | No definition | Non-invasive determination of body-water composition using BIS is feasible in critically ill patients but requires knowledge of the patient’s weight |
| Samoni et al. [11] | 2016 | Prospective, dual-center, clinician-blinded, observational study | Mixed (medical-surgical) ICU patients | 125 | To assess the impact of hyperhydration on ICU mortality in critically ill patients, comparing its measurement by BIVA and by CFB recording | Hydration > 74.3% of lean body mass (BIVA) or > 5% fluid overload (CFB) | The hydration status measured by BIVA seems to predict mortality risk in ICU patients better than the conventional method of fluid balance recording |
| Tierens et al. [21] | 2017 | Restrospective study | Mixed ICU patients | 101 | To assess the prognostic value of fluid overload in the first week of ICU-stay | 5% increment in volume excess divided by initial body weight | Higher mortality rate in ICU patients with FO was observed. FO seems a new and independent prognostic factor |
| Hize and Gonzalez [6] | 2018 | Prospective study | Critically ill patients with AKI | 224 | Assessment of the hydration status measured using BIVA in critical patients in intensive care at the time of AKI diagnosis and its association with mortality | No definition | Greater hydration present at that time of AKI diagnosis was associated with lower survival |
| Kammar-Garcia et al. [12] | 2018 | Prospective observational study | Emergency Department (ED) patients | 109 | To investigate the association of fluid overload, measured by BIVA and also by accumulated fluid balance, with 30-day mortality rates in patients admitted to the emergency department | No definition | Fluid overload on admission evaluated by BIVA, but not by accumulated FB, was significantly related to mortality in patients admitted to an ED |
| Marino et al. [14] | 2018 | Prospective study | Children after cardiac surgery | 50 | To investigate the predictive value of a preoperative measures of BIS PA 200/5° in children admitted to pediatric ICU following cardiac surgery | NA | A statistically significant relationship between BIS PA 200/5° and PICU LOS and positive fluid balance the day following surgery was found |
| Slobod et al. [2] | 2019 | Prospective observational study | Mixed medical-surgical ICU patients on mechanical ventilation | 36 | To investigate the effect of BIA-measured volume status on duration of mechanical ventilation, 28-day mortality, and AKI requiring RRT in a population of medical/surgical patients admitted to the ICU | ECW/TBW ratio > 39% | A higher ECW/TBW ratio within 24 h of admission is associated with an increase in number of ventilator days in mechanically ventilated patients |
| Razzera et al. [13] | 2019 | Prospective cohort study | Mixed (medical-surgical) ICU patients | 89 | To evaluate the validity of BIA parameters as predictors of nutrition risk and clinical outcomes in critically ill patients | BIVA values above the 75th percentile in the tolerance ellipse | (1) PA < 5.5 showed an accuracy of 79% to identify patients at high nutrition risk. (2) Hyperhydration (BIVA > 70%) was a significant predictor of mortality |
NA not applicable, AKI acute kidney injury, BCM body cell mass, BIA bio-electrical impedance analysis, BIS bioimpedance spectroscopy, BIVA bio-electrical impedance vector analysis, CFB cumulative fluid balance, CVVH continuous venovenous hemofiltration, CVP central venous pressure, FFM fat free mass, FO fluid overload, IAP intra-abdominal pressure, LOS length of stay, MF multi frequency, LOS length of stay, PA phase angle, RRT renal replacement therapy
Fig. 1.
The future of bio-electrical impedance analysis (BIA)
Shedding new light
We read with great interest the work by Ciumanghel et al. [23]. Their study highlights an important clinical problem, fluid overload assessment, in a field that lacks clinical data. The study population was appropriate as abdominal surgery is among the most common elective surgical procedures [24]. The results confirm the potential role and usefulness of BIA monitoring to quantify body fluid composition and intercompartmental shifts in a major abdominal surgery perioperative setting. In this study 71 adult patients undergoing elective major abdominal surgery were included. Patients were then divided in two subgroups according to the presence of pre- and postoperative cumulative fluid overload (CFO): Normal Hydration (NH) subgroup with CFO < 5%, and Fluid Overload (FO) subgroup with CFO > = 5%. The authors found some differences between the subgroups regarding peroperative and postoperative parameters like median surgery duration and mean fluid infusion rate, amongst others. Positive intraoperative fluid balance (2.4 ± 1.0 L) resulted in a significant increase of TBW (1.4 ± 2.4 L) and of ECW (1.4 ± 1.2 L). Intraoperative fluid balance significantly correlated with TBW change (r = 0.23, p = 0.04) and with AFO change (r = 0.31, p < 0.01). A significant correlation was found between pre- and postoperative AFO and RFO on one hand, and ICU-LOS on the other. In addition, we would like to provide some additional comments as food for further thought.
First of all, before surgery, patients in the FO subgroup had significantly lower haemoglobin values and significantly lower diastolic blood pressure than patients in the NH subgroup. Furthermore, patients in the FO more frequently underwent duodeno-pancreatectomy, esophagectomy and aorto-femoral bypass. This was the case in around 72.7% (16 out of 22 patients) in the FO subgroup. This form of extensive surgery usually concerns patients presenting with a serious illness like cancer or peripheral vessel disease, also having more comorbidities.
Second, postoperative serum albumin levels dropped dramatically in FO patients, while in NH patients it decreased to a much lesser extent. A drop of serum albumin levels in the early postoperative period is known to be multifactorial: altered metabolism, blood loss/dilution and capillary leakage-related redistribution into the third space [25–29]. The latter being probably the most important mechanism [25], which may account for > 75% of albumin decrease in the early postoperative phase. This significant parameter also appears to be related to the magnitude of postsurgical systemic inflammatory response [26, 27], which, in turn, is directly related to the extent of surgery [25] and is believed to contribute to the risk of developing postoperative complications [28, 29]. Labgaa et al. [25] identified a serum albumin decrease ≥ 10 g/L on postoperative day 1 after elective abdominal surgery to be independently associated with a threefold increased risk for postoperative complications. Therefore, we believe that serum albumin drop in FO subgroup not only mirrors a higher intraoperative fluid regimen but may also indicates a more pronounced postsurgical systemic inflammatory response in those patients.
Altogether, those points led us to the feeling that preoperatively, FO subgroup patients may have been already more ill and therefore may have required increased fluid infusion during surgery. Indeed, even if blood loss was the same in both subgroups, FO patients received significantly more colloid infusion. Data on the inotropic and vasoactive medication use were collected but are not presented in the paper. One could expect more vasopressors in the FO subgroup. However, in order to maintain patient’s blood pressure peroperatively, one can give intravenous fluid infusion or choose vasopressor drugs. Both strategies being widely accepted in clinical practice. Unfortunately, little is known about the fluid management protocol, or whether it recommends early or late use of vasopressors? Even if fluid overload is known to impair the patient's clinical course in view of aforementioned comments, the greater rate of postoperative respiratory dysfunction and longer ICU stay in FO patients [30–32] seen in the work of Ciumanghel et al., may not be explained only by the presence of fluid overload [23].
Third, concerning BIA parameters, a comparison of pre- and postoperative BIA parameters was done for the whole group, and then in FO and NH subgroups separately. All these analyses consistently showed a significant increase in TBW, ECW, ECW/ICW ratio, AFO and RFO. We would also suggest a comparative analysis between FO and NH subgroup, as we believe that the increase in BIA-parameters in the FO subgroup would be more pronounced, due both to longer surgery and more severe illness, as partially discussed above. Moreover, the preoperative AFO and RFO values (more refined BIA parameters not related to body weight or height) were significantly higher in FO group. This could, therefore, additionally indicate that NH and FO patients did not present with the same baseline parameters before surgery.
Fourth, regarding AFO and RFO differences between FO and NH patients, we were surprised to observe that FO patients had lower TBW and ECW values preoperatively compared to NH patients. This might be explained by significantly higher BMI values in NH patient’s subgroup. Hence, it would seem appropriate to present BIA data as litre/kg body weight, as it would give a more appropriate estimation of patient’s true fluid composition.
Fifth, as the authors stated themselves, having only one postoperative BIA measurement is a limitation for a broader understanding of dynamic changes in body fluid composition. More prolonged changes in the fluid balance and or distribution could probably have been revealed if later measurements would have been performed. For instance, a BIA measurement 12 and 24 h after ICU admission would have been of great interest. Nevertheless, we recognise that this was probably challenging in the particular setting of this study, as the patients in the NH subgroup (i.e., 2/3 of included patients) had a median ICU stay of 5 h. This could be an interesting topic for further studies in the field.
Take home message
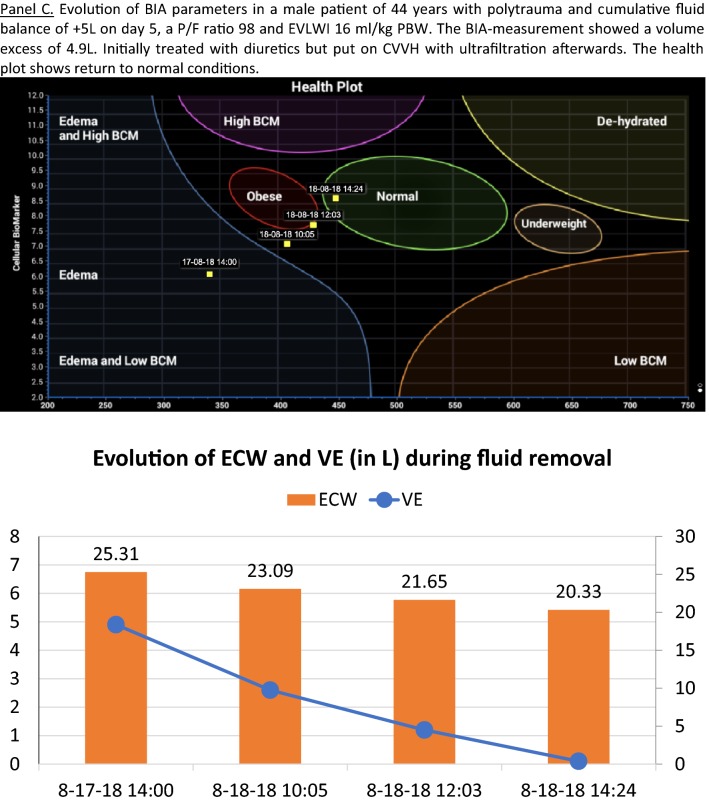
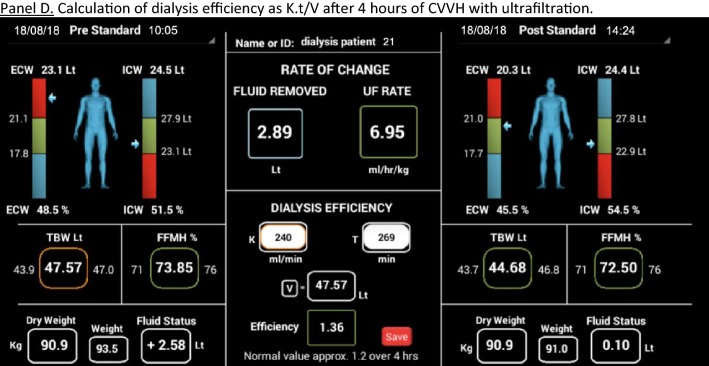
In conclusion, the study of Ciumanghel et al. addresses an important clinical problem and proposes a non-invasive, feasible, easy to perform bedside BIA-technique to assess and monitor fluid status and fluid distribution in the perioperative period, which may be of great interest to help physicians to improve management, care and outcome in critically ill patients. In the future, newer techniques may become available that allow not only calculation of TBW, ICW, ECW but also IVF and EVF. This could be of interest to assess performance of dialysis (Kt/V) but also to assess pharmacokinetics and pharmacodynamics of drugs and the fluids they are diluted in. The use of BIA in critically ill patients sounds promising but is probably not ready yet for prime time.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Basso F, Berdin G, Virzì GM, et al. Fluid management in the intensive care unit: bioelectrical impedance vector analysis as a tool to assess hydration status and optimal fluid balance in critically ill patients. Blood Purif. 2013;36(3–4):192–199. doi: 10.1159/000356366. [DOI] [PubMed] [Google Scholar]
- 2.Slobod D, Yao H, Mardini J, et al. Bioimpedance-measured volume overload predicts longer duration of mechanical ventilation in intensive care unit patients. Can J Anaesth. 2019;66(12):1458–1463. doi: 10.1007/s12630-019-01450-4. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg AL, Dechert RE, Park PK, Bartlett RH. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;24:35–46. doi: 10.1177/0885066608329850. [DOI] [PubMed] [Google Scholar]
- 4.Dewitte A, Carles P, Joannès-Boyau O, et al. Bioelectrical impedance spectroscopy to estimate fluid balance in critically ill patients. J Clin Monit Comput. 2016;30(2):227–233. doi: 10.1007/s10877-015-9706-7. [DOI] [PubMed] [Google Scholar]
- 5.Mehta RL, Clark WC, Schetz M. Techniques for assessing and achieving fluid balance in acute renal failure. Curr Opin Crit Care. 2002;8(6):535–543. doi: 10.1097/00075198-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Hise ACDR, Gonzalez MC. Assessment of hydration status using bioelectrical impedance vector analysis in critical patients with acute kidney injury. Clin Nutr. 2018;37(2):695–700. doi: 10.1016/j.clnu.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Bracco D, Revelly JP, Berger MM, et al. Bedside determination of fluid accumulation after cardiac surgery using segmental bioelectrical impedance. Crit Care Med. 1998;26(6):1065–1070. doi: 10.1097/00003246-199806000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Jones SL, Tanaka A, Eastwood GM, et al. Bioelectrical impedance vector analysis in critically ill patients: a prospective, clinician-blinded investigation. Crit Care. 2015;12(19):290. doi: 10.1186/s13054-015-1009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dabrowski W, Kotlinska-Hasiec E, Schneditz D, et al. Continuous veno-venous hemofiltration to adjust fluid volume excess in septic shock patients reduces intra-abdominal pressure. Clin Nephrol. 2014;82(1):41–50. doi: 10.5414/CN108015. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Buyun W, Gong D, et al. Fluid overload at start of continuous renal replacement therapy is associated with poorer clinical condition and outcome: a prospective observational study on the combined use of bioimpedance vector analysis and serum N-terminal pro-B-type natriuretic peptide measurement. Crit Care. 2015;19:135. doi: 10.1186/s13054-015-0871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Samoni S, Vigo V, Reséndiz LI, et al. Impact of hyperhydration on the mortality risk in critically ill patients admitted in intensive care units: comparison between bioelectrical impedance vector analysis and cumulative fluid balance recording. Crit Care. 2016;8(20):95. doi: 10.1186/s13054-016-1269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kammar-García A, Pérez-Morales Z, Castillo-Martinez L, et al. Mortality in adult patients with fluid overload evaluated by BIVA upon admission to the emergency department. Postgrad Med J. 2018;94(1113):386–391. doi: 10.1136/postgradmedj-2018-135695. [DOI] [PubMed] [Google Scholar]
- 13.Razzera EL, Marcadenti A, Rovedder SW, et al. parameters of bioelectrical impedance are good predictors of nutrition risk, length of stay, and mortality in critically Ill patients: a prospective cohort study. J Parenter Enter Nutr. 2019 doi: 10.1002/jpen.1694. [DOI] [PubMed] [Google Scholar]
- 14.Marino LV, Griksaitis MJ, Pappachan JV. Preoperative bioelectrical impedance predicts intensive care length of stay in children following cardiac surgery. Cardiol Young. 2018;28(5):779–782. doi: 10.1017/S1047951118000136. [DOI] [PubMed] [Google Scholar]
- 15.Piccoli A, Pittoni G, Facco E, et al. Relationship between central venous pressure and bioimpedance vector analysis in critically ill patients. Crit Care Med. 2000;28(1):132–137. doi: 10.1097/00003246-200001000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Ismael S, Savalle M, Trivin C, et al. The consequences of sudden fluid shifts on body composition in critically ill patients. Crit Care. 2014;18(2):R49. doi: 10.1186/cc13794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee H, Jang KS, Shin MJ, et al. Use of multifrequency bioimpedance analysis in male patients with acute kidney injury who are undergoing continuous veno-venous hemodiafiltration. PLoS ONE. 2015;10(7):e0133199. doi: 10.1371/journal.pone.0133199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.House A, Haapio M, Lentini P, et al. Volume assessment in mechanically ventilated critical care patients using bioimpedance vectorial analysis, brain natriuretic peptide, and central venous pressure. Int J Nephrol. 2010;2(2011):413760. doi: 10.4061/2011/413760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mól N, Kwinta P. Assessment of body composition using bioelectrical impedance analysis in preterm neonates receiving intensive care. Dev Period Med. 2015;19(3 Pt 1):297–304. [PubMed] [Google Scholar]
- 20.Lingwood BE, Coghlan JP, Ward LC, et al. Measurement of extracellular fluid volume in the neonate using multiple frequency bio-impedance analysis. Physiol Meas. 2000;21(2):251–262. doi: 10.1088/0967-3334/21/2/305. [DOI] [PubMed] [Google Scholar]
- 21.Tierens S, Noori H, Gilleman M, et al. Assessment of fluid overload in ICU patients prognostic value of bioelectrical impedance analysis. Anaesthesiol Intensive Ther. 2019;Suppl. 1:48–50. doi: 10.5114/ait.2021.103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malbrain ML, Huygh J, Dabrowski W, et al. The use of bio-electrical impedance analysis (BIA) to guide fluid management, resuscitation and deresuscitation in critically ill patients: a bench-to-bedside review. Anaesthesiol Intensive Ther. 2014;46(5):381–391. doi: 10.5603/AIT.2014.0061. [DOI] [PubMed] [Google Scholar]
- 23.Ciumanghel AI, Grigoras I, Siriopol D, et al. Bio-electrical impedance analysis for perioperative fluid evaluation in open major abdominal surgery. J Clin Monit Comput. 2019 doi: 10.1007/s10877-019-00334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noordzij PG, Poldermans D, Schouten O, et al. Postoperative mortality in The Netherlands: a population-based analysis of surgery-specific risk in adults. Anesthesiology. 2010;112(5):1105–1115. doi: 10.1097/ALN.0b013e3181d5f95c. [DOI] [PubMed] [Google Scholar]
- 25.Labgaa I, Joliat GR, Kefleyesus A, et al. Is postoperative decrease of serum albumin an early predictor of complications after major abdominal surgery? A prospective cohort study in a European centre. BMJ Open. 2017;7(4):e013966. doi: 10.1136/bmjopen-2016-013966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hübner M, Mantziari S, Demartines N, et al. Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome: a pilot study. Gastroenterol Res Pract. 2016;2016:8743187. doi: 10.1155/2016/8743187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smeets HJ, Kievit J, Dulfer FT, et al. Analysis of post-operative hypalbuminaemia: a clinical study. Int Surg. 1994;79:152–157. [PubMed] [Google Scholar]
- 28.Kohl BA, Deutschman CS. The inflammatory response to surgery and trauma. Curr Opin Crit Care. 2006;12:325–332. doi: 10.1097/01.ccx.0000235210.85073.fc. [DOI] [PubMed] [Google Scholar]
- 29.Amar D, Zhang H, Park B, et al. Inflammation and outcome after general thoracic surgery. Eur J Cardiothorac Surg. 2007;32:431–434. doi: 10.1016/j.ejcts.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 30.O’Connor ME, Prowle JR. Fluid Overload. Crit Care Clin. 2015;31(4):803–821. doi: 10.1016/j.ccc.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Cordemans C, De Laet I, Van Regenmortel N, et al. Fluid management in critically ill patients: the role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann Intensive Care. 2012;2(1):S1. doi: 10.1186/2110-5820-2-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malbrain ML, Marik PE, Witters I, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46(5):361–380. doi: 10.5603/AIT.2014.0060. [DOI] [PubMed] [Google Scholar]