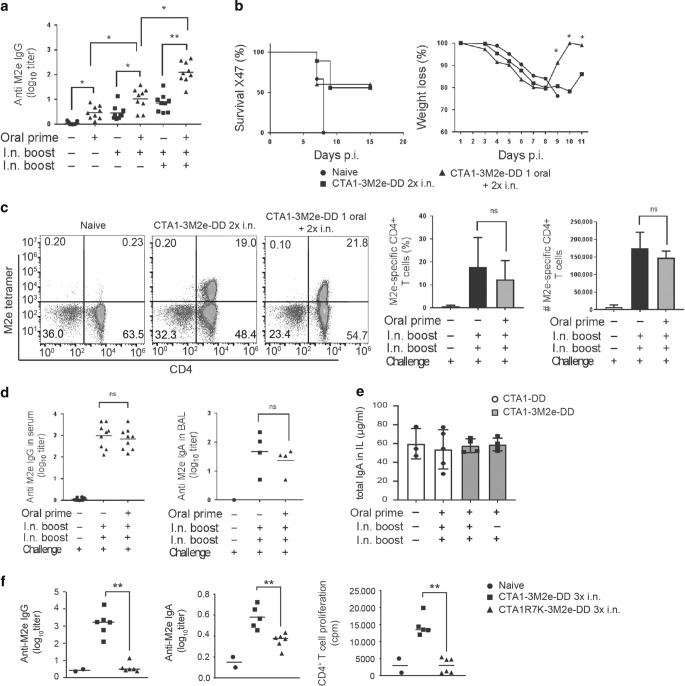
Fig. 6. Reduced morbidity to influenza infection in orally CTA1-3M2e-DD primed neonatal mice.
Groups of ten mice were given PBS or primed p.o. with 10 μg of CTA1-3M2e-DD followed by two intranasal immunizations with 10 μg of CTA1-3M2e-DD and 15 days later the mice were challenged by 1 × LD50 of a mouse adapted X47 influenza A virus strain. Neonatal mice were primed p.o. at post natal day 5 and received one or two i.n. booster doses with CTA1-3M2e-DD at 10-day intervals, as indicated. Control mice received only i.n immunizations with CTA1-3M2e-DD without the p.o. priming. a Anti-M2e IgG antibodies in serum were assessed after one, two, or three immunizations with CTA1-3M2e-DD without or with (d) a virus challenge infection. b Percentage of surviving animals and the monitored changes of body weight following an X47-challenge infection. c Representative FACS plots of M2e-tetramer-specific lung CD4 T cells isolated from mice immunized with CTA1-3M2e-DD, as indicated. Analysis of the frequency and absolute number M2e-specific CD4 T cells of all CD4 T cells in the lungs. d Anti-M2e IgG in serum and IgA in BAL after a challenge infection. e Total IgA in intestinal lavage after oral priming and i.n boosting with CTA1-3M2e-DD or CTA1-DD in neonatal mice, as indicated. f M2e-specific IgG and IgA titers in serum, and CD4+ T-cell proliferation to recall antigen in the mediastinal lymph node of adult mice following three i.n. doses of CTA1-3M2e-DD or the enzymatically inactive CTA1R7K-3M2e-DD. Statistical analysis was performed using two-way ANOVA followed by Dunnet correction for multiple comparison; *p < 0.05, **p < 0.01.