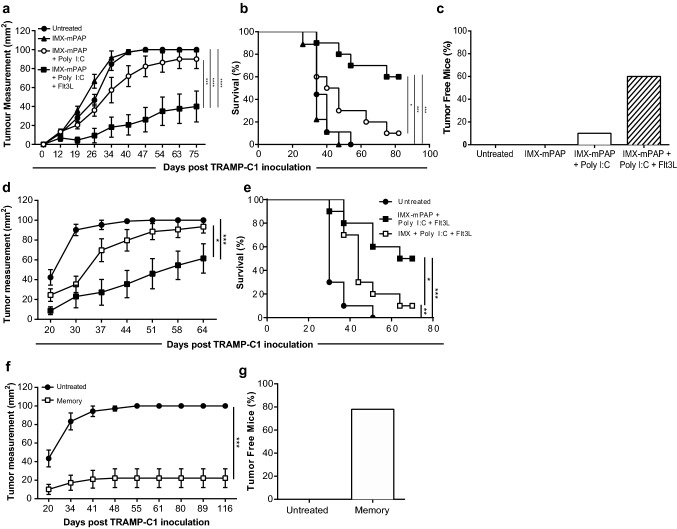
Fig. 1.
ISCOMATRIX™ adjuvant formulated with mPAP, Poly I:C and Flt3L provides therapeutic protection against TRAMP-C1 tumors. Animals that were untreated were compared to animals that were vaccinated with either, IMX–mPAP, IMX–mPAP + pIC or IMX–mPAP + pIC + Flt3. Day 2 prime and day 9 boost (a–c). a Tumor growth. b Percent survival. c Tumor free mice at conclusion of experiment. d, e Mice vaccinated with IMX + pIC + Flt3 plus or minus antigen-mPAP were compared for d growth of TRAMP-C1 tumor and e percent survival. f, g Mice previously challenged with TRAMP-C1 tumors that had received the combination vaccine IMX–mPAP + pIC + Flt3L, that had become tumor free and had remained so for at least 90 days, were rechallenged with TRAMP-C1 cells. f Tumor growth in protected animals was compared to age-matched naïve mice inoculated with TRAMP-C1 g Tumor free mice. Data are presented as mean ± SEM where n = 10 mice/group from one representative experiment of three equivalent experiments (a–e) or where n = 10 (Untreated) and 18 (memory) (f, g) mice/group from one representative experiment of two equivalent experiments. Statistical significance in tumor growth (a, d, f) was determined using a two-way ANOVA with Tukey’s multiple comparisons test. Percent survival (b, e) was plotted as a Kaplan–Meier curve and the log-rank (Mantel–Cox) test was used to calculate statistical significance. p < 0.05*, p < 0.01**, p < 0.001*** and p < 0.0001****. IMX = ISCOMATRIX® adjuvant