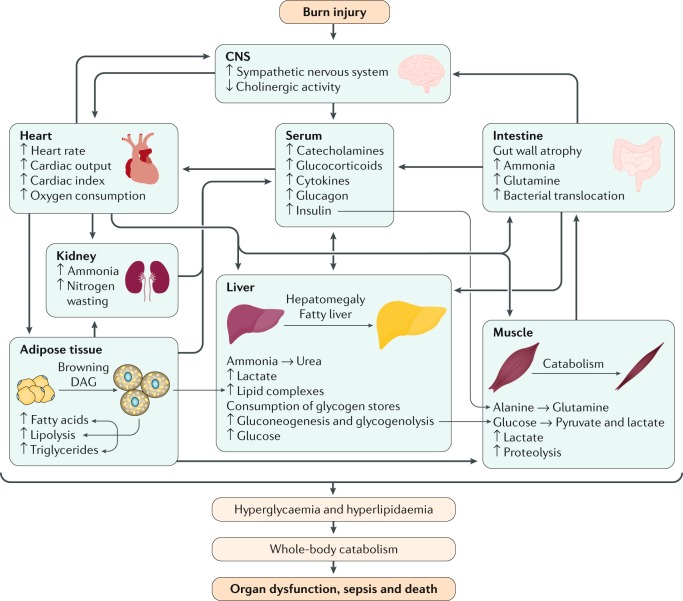
Fig. 3. Hypermetabolic state in burn injury.
Severe burn injury induces a unique and remarkably complex response that involves the release of stress hormones and pro-inflammatory mediators. The immediate response leads to a hypometabolic response that lasts for ~72–96 hours (ebb phase), but then rapidly turns into the flow phase that can persist for years after the initial injury. Stress mediators, such as catecholamines, glucocorticoids and cytokines, are released into the system and cause a plethora of systemic responses. The heart goes into a hyperdynamic overdrive, increasing circulation and blood flow to increase oxygen and nutrient delivery. However, increased stress signalling causes changes in organ function and metabolic demand. Protein is degraded to deliver energy for hepatic function, the gut develops mucosal atrophy to absorb more nutrients but also enabling bacterial translocation. The kidneys are hyperperfused but oxygen delivery is decreased, leading to acute kidney injury and stress signals from the kidney. The interplay between these organs accumulates, leading to metabolic and inflammatory overdrive that subsequently causes white adipose tissue to change to brown adipose tissue. Brown adipose tissue releases energy and induces substantial lipolysis with the accompanying expression of lipotoxic intermediates, such as triglycerides, free fatty acids and diacylglycerols (DAG), all of which are transferred to the liver. The liver is unable to metabolize all of the accumulating substances and develops hepatomegaly. In turn, hyperlipidaemia and hyperglycaemia with insulin resistance is present, which worsens the hypermetabolic and inflammatory state. If hypermetabolism cannot be diminished or decreased, holistic catabolism ensues and, subsequently, multiple organ failure and death. CNS, central nervous system.