Abstract
Background
Specific prognostic models for intracerebral hemorrhage (ICH) have short and simple features, whereas intensive care unit (ICU) severity scales include more complicated parameters. Even though newly developed ICU severity scales have disease-specific properties, they still lack radiologic parameters, which is crucial for ICH.
Aims
To compare the performance of the Simplified Acute Physiology Score (SAPS) III, Acute Physiology and Chronic Health Evaluation (APACHE) IV, Logistic Organ Dysfunction Score (LODS), ICH, max-ICH, ICH functional outcome score (ICH-FOS), and Essen-ICH for prediction of in-hospital and one-year mortality of patients with ICH.
Methods
A single-center analysis of 137 patients with ICH was conducted over 5 years. The performance of scoring systems was evaluated with receiver operating characteristic analysis. The independent predictors of one-year mortality were investigated with a multivariate logistic regression analysis. The SAPS-III score was calculated both in the emergency department (ED) and ICU.
Results
Among the independent variables, the need for mechanical ventilation, hematoma volume, the presence of intraventricular hemorrhage, and hematoma originating from both lobar and nonlobar regions were found as the strongest predictor of one-year mortality. For in-hospital mortality, the discriminative power of SAPS-II, APACHE-IV, and LODS was excellent, and for SAPS-III-ICU and SAPS-III-ED, it was good. For one-year mortality, the discriminative power of SAPS-II, APACHE-IV, LODS, and SAPS-III-ICU was good, and for SAPS-III-ED, Essen-ICH, ICH, max-ICH, and ICH-FOS, it was fair.
Conclusions
Although all three ICH-specific prognostic scales performed satisfactory results for predicting one-year mortality, the common intensive care severity scoring showed better performance. SAPS-III scores may be recommended for use in EDs after proper customization.
Electronic supplementary material
The online version of this article (10.1007/s12028-020-00987-3) contains supplementary material, which is available to authorized users.
Keywords: APACHE, Cerebral hemorrhage, Mortality, Patient outcome assessment, Prognosis, Simplified acute physiology score, Stroke
Introduction
Spontaneous, nontraumatic intracerebral hemorrhage (ICH) caused by bleeding into parenchymal brain tissue is a subtype of stroke. ICH is thought to be responsible for 9–27% of all strokes, and it is related to poor outcomes [1–3].
Scoring systems enable a common language for communication between physicians. Estimating prognosis and long-term functional outcome is essential for guiding the treatment of ICH, and it is strongly recommended to perform a baseline severity score as a part of the initial evaluation [4]. Severity scoring systems are also routinely used in intensive care units (ICUs) for decision-making about treatment and to ensure the effective use of ICU beds.
Many prognostic scores have been used to predict the short- and long-term outcomes of ICH patients, but none is an optimal scale alone. The ICH score was the first developed and the most widely used clinical grading scale [5]. However, numerous studies have shown the superiority of max-ICH score, ICH functional outcome score (ICH-FOS), and Essen-ICH score to the ICH score [6–11]. The Acute Physiology and Chronic Health Evaluation (APACHE) IV is the latest version of the APACHE-II scale, which was modified by adding a new set of parameters and disease-specific subgroups [12]. Although the Logistic Organ Dysfunction Score (LODS) and Simplified Acute Physiology Score (SAPS) II are older scales in comparison with APACHE-IV, they are still frequently used in most ICUs [13, 14].
Specific prognostic models for patients with ICH have short and simple features. Still, they are limited by using neurologic examinations and radiologic findings without metabolic and physiologic parameters on initial arrival. On the other hand, despite the disease-specific structure and the contents of physiologic parameters of newly developed ICU severity scales, radiologic findings were not featured in this classification, which is crucial for ICHs [15].
The aim of this study was to compare the performances of frequently used severity scores, ICH, max-ICH, ICH-FOS, Essen-ICH, SAPS-II, LODS, and APACHE-IV, and to predict short- (in-hospital) and long-term (one-year) mortality rates in patients with ICH.
Materials and Method
Patient Selection
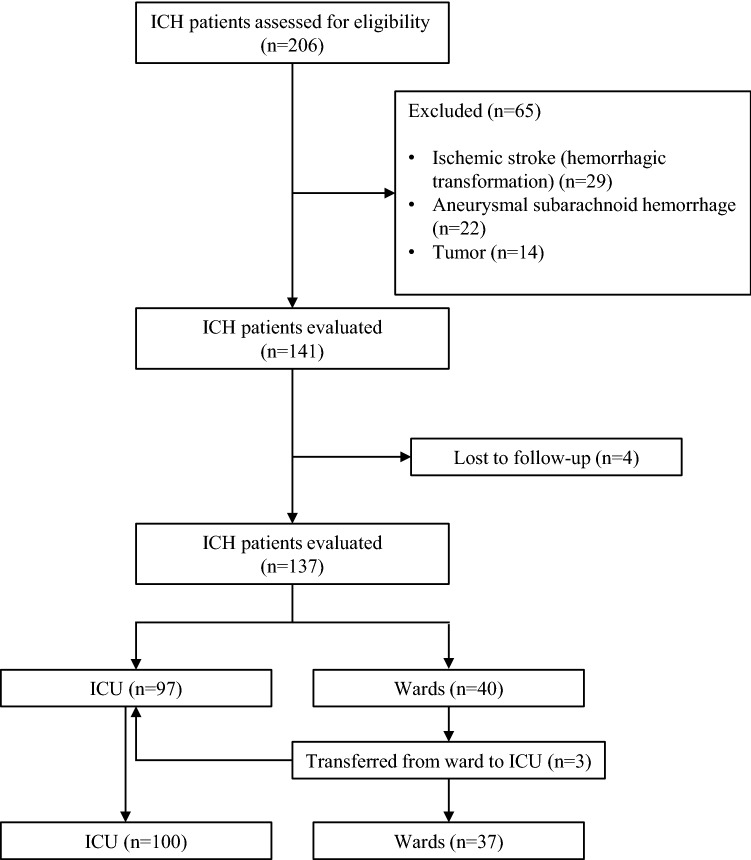
After approval of our institute’s ethical committee, this study was conducted in a tertiary care hospital, Derince Training, and Research Hospital. The medical records of patients with spontaneous nontraumatic ICH who were treated in the ICU, neurology, and neurosurgery wards between 2013 January and 2018 December were analyzed retrospectively. Data collection included age, sex, previous medical and surgical history, the use of anticoagulants, ICU admission, length of ICU stay, length of hospital stay (ICU and ward), and short- and long-term outcomes. Patients aged 18 years or older with primary spontaneous nontraumatic ICH were included in the study. Patients with secondary ICH caused by a tumor, brain structural abnormalities (aneurysms and arteriovenous malformations), trauma, hemorrhagic transformation of acute ischemic stroke, and patients with incomplete data and those lost to follow-up were excluded from the study.
Emergency Department
Computed tomography (CT) was performed as soon as possible after admission to the emergency department (ED). Following the diagnosis of ICH, treatment including blood pressure lowering and reversal of anticoagulants was initiated immediately. Glasgow Coma Scale (GCS) scores and laboratory parameters on admission were recorded. The National Institutes of Health Stroke Scale (NIHSS) scores were calculated according to the physical examination by neurology specialists. Additionally, the SAPS-III score was calculated at the first hour of admission. Patients were transferred to either the ICU, neurosurgery or neurology wards according to the clinical condition after the first-line medical care in the ED.
Surgical Management
The decision for a primary emergency decompressive surgery was made according to the patient’s physical and medical condition. Burr hole trephination, craniotomy, or decompressive craniectomy procedures were used for the evacuation of hematomas. An external ventricular drain (EVD) was surgically inserted if hydrocephalus had developed in the presence of intraventricular hemorrhage.
Intensive Care Unit
Patients who were hemodynamically unstable, required mechanical ventilation, deteriorated rapidly, had GCS scores lower than 14, and underwent surgery were transferred to the ICU for close follow-up. All patients’ biochemistry, hemograms, and arterial blood gases were analyzed on admission to the ICU and repeated in necessary and appropriate conditions. Blood pressure-lowering therapy was administered to patients with elevated systolic blood pressure. Vasopressor therapy was initiated if the patient was hypotensive even though hypovolemia was corrected. Patients were transferred to palliative care, neurology, or neurosurgery wards after ICU treatment.
Long-Term Follow-Up
The data of survival at 12 months after the onset of ICH were obtained from the national death registry database; it is compulsory, legally, to record every death in the country in this system [16].
Calculation of Prognostic Scores
The ICH, max-ICH, ICH-FOS, Essen-ICH, and SAPS-III-ED scores were calculated as previously described for all patients with ICH [5–8].
The LODS, APACHE-IV, SAPS-II, SAPS-III-ICU, and mortality rates were calculated for the patients only with ICU admission. The SAPS-III-ED score was calculated for all patients with ICH in the ED, depending on the variables within the first hour of admission to the hospital. The SAPS-III-ICU score was recalculated for patients with ICH admitted to the ICU depending on the worst values within the first hour in the ICU.
Evaluation of Radiographic Features
All brain CT imaging was evaluated by a neurologist and anatomist who were involved in this study. Hematoma volume was calculated in milliliters using the ABC/2 formula without taking into account the intraventricular hemorrhage volume [17, 18]. Midline shift was measured in millimeters as the perpendicular distance between septum pellucidum, and a midline was drawn between crista frontalis and protuberentia occipitalis interna (representing falx cerebri). The localization and extension of ICH were assessed as previously described [19]. Lobar ICH included ICH involving the cortex and cortical–subcortical junction, whereas deep ICH was defined as ICH originating from the thalamus, basal ganglia, capsula interna, and deep periventricular white matter. Nonlobar ICH included deep and infratentorial origin [19].
Statistics
All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) Ver. 20 (IBM Corp., USA). The selected test for normal distribution was the Shapiro–Wilk test. Normally distributed continuous variables were analyzed using Student’s t test and presented with mean and standard deviation (± SD). Non-normally distributed continuous variables were tested using the Mann–Whitney U test and presented with median and interquartile range (IQR). Categorical variables were tested using the Chi-square and Fisher’s exact tests. In addition, receiver operating characteristic (ROC) curve analysis was conducted to find out optimal cutoff levels of risk scores. The performance of the tests according to the area under the ROC curve (AUC) was evaluated as previously described [20]. The sensitivity and specificity of the demonstrated cutoff values are presented.
The independent predictors of one-year mortality were investigated with a logistic regression model. The variables that were found as statistically significant were included in multivariate analysis. Before the final analysis, a multicollinearity analysis was performed to exclude correlated variables (r > 0.5). The effect sizes are presented as odds ratios (ORs) and 95% confidence intervals (CIs). The fitness of the model was tested using the Hosmer–Lemeshow test. For the whole analysis, an alpha value of 0.05 was accepted as the nominal level of significance.
Results
Patient Characteristics
A total of 206 patients with nontraumatic ICH were assessed for eligibility; 69 patients (29 with hemorrhagic transformation of acute ischemic stroke, 22 with aneurysmal subarachnoid hemorrhage, 14 with underlying tumors, and four with missing records) were excluded from further analysis (Fig. 1). The mean age of all patients was 64.8 ± 14.3 years, and 56.9% were male. The rate of in-hospital mortality was 42.3% (n = 58), and the rate of one-year mortality was 51.1% (n = 70). The remaining evaluated baseline characteristics, including medical and surgical history, medications, clinical, surgical, and radiologic features of the patients, are listed in Table 1. Among these variables, parameters with p < 0.05 were included in the multivariable logistic regression analysis model for one-year mortality. Among the correlated variables (p < 0.05 and r > 0.5 in multicollinearity analysis), independent variables that had the largest effect size were included in the multivariable logistic regression model. Accordingly, age, coronary artery disease, warfarin use, NIHSS, blood glucose level, the need for mechanical ventilation, hematoma volume, intraventricular hemorrhage, EVD, and the origin of hematoma (lobar, nonlobar, both lobar and nonlobar) were found to be independent predictors of one-year mortality in ICH (Table 2). The Hosmer–Lemeshow test indicated the model fitted well (p = 0.931). The need for mechanical ventilation, hematoma volume, intraventricular hemorrhage, and both lobar and nonlobar origin showed statistical significance, and these variables were found as the strongest independent predictors of one-year mortality. All causes of in-hospital mortality are presented in Supplemental Table.
Fig. 1.
Flowchart of patient selection
Table 1.
Patient characteristics according to one-year outcome
| All patients (n = 137) | Death (n = 70) | Survival (n = 67) | p | |
|---|---|---|---|---|
| Age | 64.8 ± 14.3 | 67.5 ± 13.7 | 62 ± 14.5 | 0.024 |
| Gender (Male) | 78 (56.9) | 44 (62.9) | 34 (50.7) | 0.152 |
| Medical history | ||||
| Atrial fibrillation | 51 (37.2) | 38 (54.3) | 13 (19.4) | < 0.001 |
| Hypertension | 121 (88.32) | 65 (92.9) | 56 (83.6) | 0.091 |
| Diabetes mellitus | 41 (29.9) | 23 (32.9) | 18 (26.9) | 0.444 |
| Chronic kidney disease | 26 (19.0) | 14 (20.0) | 12 (17.9) | 0.755 |
| Chronic obstructive pulmonary disease | 13 (9.5) | 9 (12.9) | 4 (6.0) | 0.169 |
| Coronary artery disease | 49 (35.8) | 34 (48.6) | 15 (22.4) | 0.001 |
| Peripheral artery disease | 18 (13.1) | 8 (11.4) | 10 (14.9) | 0.545 |
| Cirrhosis | 4 (2.9) | 2 (2.9) | 2 (3.0) | 1.000 |
| Cancer | 7 (5.1) | 3 (4.3) | 4 (6.0) | 0.714 |
| Previous hemorrhage | 13 (9.5) | 5 (7.1) | 8 (11.9) | 0.338 |
| Previous ischemia | 58 (42.3) | 35 (50.0) | 23 (34.3) | 0.063 |
| Surgical history | ||||
| Coronary angiography | 33 (24.1) | 21 (30.0) | 12 (17.9) | 0.098 |
| Coronary bypass grafting | 17 (12.4) | 12 (17.1) | 5 (7.5) | 0.086 |
| Heart valve replacement | 7 (5.1) | 4 (5.7) | 3 (4.5) | 1.000 |
| Antiplatelet and anticoagulant use | ||||
| Acetylsalicylic acid | 66 (48.2) | 34 (48.6) | 32 (47.8) | 0.924 |
| Warfarin | 19 (13.9) | 15 (21.4) | 4 (6.0) | 0.009 |
| Clopidogrel | 5 (3.7) | 4 (5.7) | 1 (1.5) | 0.366 |
| Parameters upon admission to emergency department | ||||
| Blood glucose | 137 (103–194) | 171 (121–221) | 118 (98–164) | < 0.001 |
| Hemoglobin | 12.8 (11.7–13.9) | 12.8 (11.4–13.9) | 12.8 (11.8–13.8) | 0.653 |
| Hematocrit | 38.9 (35.0–41.9) | 38.7 (34.4–42.0) | 39 (35.6–41.8) | 0.504 |
| GCS | 13 (8–14) | 9 (6–14) | 14 (13–15) | < 0.001 |
| NIHSS | 12 (6–20) | 19 (11–22) | 8 (4–12) | < 0.001 |
| Radiographic features | ||||
| Supratentorial | 122 (89.1) | 61 (87.1) | 61 (91.0) | 0.366 |
| Infratentorial | 13 (9.5) | 7 (10.0) | 6 (9.0) | |
| Both supra and infratentorial | 2 (1.5) | 2 (2.9) | 0 (0.0) | |
| Lobar | 51 (38.0) | 20 (28.6) | 31 (46.3) | 0.016 |
| Nonlobar | 68 (49.6) | 37 (52.9) | 31 (46.3) | |
| Both lobar and nonlobar | 18 (13.1) | 13 (18.6) | 5 (7.5) | |
| Intraventricular hemorrhage | 50 (36.5) | 42 (60.0) | 8 (11.9) | < 0.001 |
| Midline shift | 4 (0–7) | 5 (0–9) | 1 (0–5) | < 0.001 |
| Hematoma volume | 15.6 (6.4–38.7) | 22.4 (10.4–48.1) | 8.5 (3.0–28.5) | < 0.001 |
| Neurosurgical interventions | ||||
| Nonsurgical | 86 (62.8) | 41 (58.6) | 45 (67.2) | 0.218 |
| Hematoma evacuation and/or decompression | 51 (37.2) | 29 (41.4) | 22 (32.9) | 0.416 |
| External ventricular drain | 15 (10.9) | 14 (20.0) | 1 (1.5) | < 0.001 |
| Status on intensive care unit admission and follow-up | ||||
| Mechanical ventilation | 78 (56.9) | 64 (91.4) | 14 (20.9) | < 0.001 |
| Timing of intubation (day) | 1 (0–1) | 1 (1–1) | 0 (0–0) | < 0.001 |
| Days on mechanic ventilator | 2 (0–13) | 11 (5–22) | 0 (0–0) | < 0.001 |
| The need for vasopressors within the first 24 h | 12 (8.8) | 12 (17.1) | 0 (0.0) | < 0.001 |
| Epilepsy | 18 (13.1) | 11 (15.7) | 7 (10.4) | 0.362 |
| Discharge status | ||||
| Total length of hospital stay | 12 (8–22) | 14 (7–32) | 12 (9–17) | 0.080 |
| Total length of ICU stay | 5 (0.0–14.0) | 11 (6.3–26.5) | 0 (0.0–5.0) | < 0.001 |
Bold values indicate statistical significance (p < 0.05)
Categorical variables are presented as n (%), continuous variables are presented as median (interquartile range 25–75) or mean ± standard deviation
GCS Glasgow Coma Scale; mRS Modified Ranking Scale; NIHSS National Institutes of Health Stroke Scale
Table 2.
Logistic regression analysis of predictors for one-year mortality of ICH patients
| Wald | Odds ratio | Confidence interval (95%) | p | |
|---|---|---|---|---|
| Age | 0.097 | 1.008 | 0.961–1.056 | 0.755 |
| Coronary artery disease | 3.039 | 4.030 | 0.841–19.312 | 0.081 |
| Warfarin use | 0.237 | 1.640 | 0.224–12.006 | 0.626 |
| NIHSS | 0.153 | 1.020 | 0.924–1.126 | 0.696 |
| Blood glucose | 2.658 | 1.008 | 0.998–1.018 | 0.103 |
| Mechanical ventilation | 18.347 | 28.549 | 6.159–132.320 | < 0.001 |
| Hematoma volume | 4.717 | 1.057 | 1.005–1.111 | 0.030 |
| Intraventricular hemorrhage | 5.758 | 5.588 | 1.371–22.785 | 0.016 |
| External ventricular drain | 0.011 | 1.150 | 0.088–14.990 | 0.915 |
| Lobar origin | 8.186 | 0.017 | ||
| Nonlobar origin | 0.720 | 0.375 | 0.039–3.610 | 0.396 |
| Both lobar and nonlobar origin | 6.645 | 15.409 | 1926–123.275 | 0.010 |
Bold values indicate statistical significance (p < 0.05)
NIHSS National Institutes of Health Stroke Scale
Predicting In-Hospital Mortality
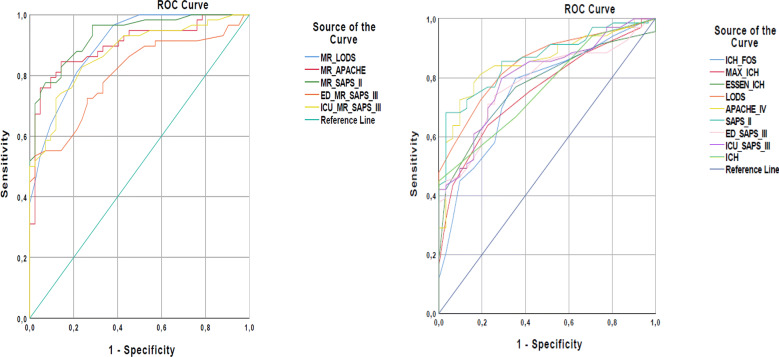
The discriminative power for in-hospital mortality of all scoring systems that calculate mortality rates was evaluated using ROC analysis. Accordingly, the discriminative power of the SAPS-II, APACHE-IV, and LODS was excellent (AUC ≥ 0.9), SAPS-III-ICU, and SAPS-III-ED was good (AUC ≥ 0.8) (Table 3). Among these scoring systems, the predicted mortality rate of SAPS-II had the highest AUC value (0.932, 95% CI 0.886–0.978). For the predicted mortality rate of SAPS-II (cutoff 23.9), sensitivity was calculated as 82.8%, and specificity was calculated as 85.7% (Fig. 2).
Table 3.
Performance of the scoring systems for predicting in-hospital mortality rate
| Predicted mortality rate | AUC (95% CI) | Sensitivity (%) | Specificity (%) | p |
|---|---|---|---|---|
| SAPS-II | 0.932 (0.886–0.978) | 82.8 | 85.7 | < 0.001 |
| APACHE-IV | 0.900 (0838–0.961) | 84.5 | 83.3 | < 0.001 |
| LODS | 0.900 (0.842–0.959) | 81.0 | 78.6 | < 0.001 |
| SAPS-III-ICU | 0.873 (0.805–0.940) | 74.1 | 85.7 | < 0.001 |
| SAPS-III-ED | 0.804 (0.720–0.889) | 72.4 | 71.4 | < 0.001 |
AUC area under the curve; CI confidence interval; ED emergency department; ICU intensive care unit
Fig. 2.
Diagnostic accuracy of the prognostic models specific for ICH and conventional ICU scoring systems. MR mortality rate
Predicting One-Year Mortality
The discriminative power for one-year mortality of all scoring systems was evaluated using ROC analysis. Accordingly, the discriminative power of SAPS-II, APACHE-IV, LODS, and SAPS-III-ICU was good (AUC ≥ 0.8), and for SAPS-III-ED, Essen-ICH, ICH, max-ICH, and ICH-FOS, it was fair (AUC ≥ 0.7) (Table 4). Among these scoring systems, SAPS-II had the highest AUC value (0.863, 95% CI 0.793–0.934). For SAPS-II (cutoff 37.5), sensitivity was calculated as 76.8% and specificity was calculated as 74.2% (Fig. 2).
Table 4.
Performance of the scoring systems for one-year mortality in ICH patients
| SAPS-II | AUC (95% CI) | Sensitivity (%) | Specificity (%) | p |
|---|---|---|---|---|
| 0.863 (0.793–0.934) | 76.8 | 74.2 | < 0.001 | |
| APACHE-IV | 0.853 (0.778–0.929) | 81.2 | 80.6 | < 0.001 |
| LODS | 0.849 (0.775–0.923) | 81.2 | 71.0 | < 0.001 |
| SAPS-III-ICU | 0.805 (0.719–0.892) | 79.7 | 71.0 | < 0.001 |
| SAPS-III-ED | 0.782 (0.691–0.872) | 73.9 | 74.2 | < 0.001 |
| Essen-ICH | 0.773 (0.681–0.864) | 76.8 | 64.5 | < 0.001 |
| ICH | 0.765 (0.674–0.857) | 66.7 | 65.5 | < 0.001 |
| Max-ICH | 0.756 (0.661–0.851) | 63.8 | 77.4 | < 0.001 |
| ICH-FOS | 0.754 (0.654–0.854) | 79.7 | 64.5 | < 0.001 |
AUC area under the curve; CI confidence interva; ED emergency department; ICU intensive care unitl
Discussion
The evolution in the development of scoring systems has gained momentum in the last decades and is still ongoing. The reason for this advance is the difficulty in agreeing criteria in each scoring system and their limited use in special patient groups. Scoring systems are used to determine the severity of the disease, and to evaluate performance in terms of general or specific disease categories, and to provide for physicians the ability to assess the prognosis and treatment schedule of patients individually [21]. Following the proposal of the ICH score in 2001, it has become the most frequently used communication tool in many institutes [5]. After this, approximately 20 grading scales for ICH have been created to date [9]. These scores generally include only the parameters obtained from radiologic and neurologic examinations. However, ICH is a condition that affects patients both neurologically and multi-systemically. Therefore, to improve outcomes, it is strongly recommended that the follow-up and treatment of patients with ICH occur in intensive care conditions or a stroke unit [22]. Additionally, it is important to keep the problem in perspective while predicting outcomes, rather than using only a neurologic evaluation.
The advantage of the SAPS-II, APACHE-IV, and LODS scores used in ICUs is the evaluation of changes in the clinical condition of patients within the first 24 h. Clinical changes are evaluated according to the worst values; accordingly, they are thought to overestimate mortality [23]. APACHE-II is now routinely used in many ICUs and has good-to-excellent discriminative power outcomes. APACHE-IV is the recent and revised version of APACHE-II, according to specific disease groups. In many specific disease groups, including stroke, APACHE-IV has been shown to be superior to APACHE-II [24]. Although APACHE-II and SAPS-II scores were not designed for specific patient groups, they have good accuracy in patients with hemorrhagic and ischemic stroke [25–27]. The SAPS-III score is based on the patient’s assessment during the first hour of ICU admission [28]. Most of the data used in the SAPS-III score are derived from information before ICU admission, so in a previous study, it was hypothesized that the SAPS-III score could be used for ICU triage [29]. Based on this idea, we calculated the SAPS-III score for patients with ICH in the first hour of admission to both the ED and ICU.
Patients with ICH can often be unstable in the first days or even in the first hours following the onset of bleeding [4, 22]. It was observed that SAPS-III-ED had lower discriminative power than SAPS-III-ICU because patients could rapidly deteriorate in the hours following admission. The performance of both SAPS-III scores calculated in the two treatment units was poor in patients with ICH when compared with other conventional intensive care severity scores. However, SAPS-III-ED and SAPS-III-ICU scores showed better performance compared with Essen-ICH, ICH, max-ICH, and ICH-FOS scores in predicting one-year mortality.
ICH is a medical emergency, and early aggressive treatment is crucial. Therefore, treatment should start in the ED and the management plan should be conducted multidisciplinarily [4]. Following the first-line treatment in ED, these patients can be transferred to the ICU, neuro-ICU, acute stroke unit or a general ward according to their general clinical status. Patients who are followed up in ICUs or acute stroke units are suggested to have better outcomes than patients in general wards [22]. However, depending on the infrastructure of the treatment center and for the effective use of intensive care beds, it may be preferable to follow up certain patients in the wards. There is no present severity scoring system currently used in EDs for the triage of patients with ICH. Considering the possibility that patients with ICH can rapidly deteriorate within hours, the SAPS-III score may be recommended for use in EDs for triage after proper customization. For example, a patient with a good clinical condition, low-volume, but infratentorial bleeding should never be directed to a ward. On the other hand, a patient with a good clinical condition and low-volume cortical bleeding can be referred to a ward. However, the interpretation of this result needs to be used cautiously in clinical practice.
The LODS is a simple intensive care severity score that evaluates organ dysfunction in the first 24 h of admission, regardless of a specific diagnosis. To our knowledge, LODS has never been used in patients with ICH. Interestingly, it was observed in this study that the LODS system, which was much more simplified compared with the other intensive care severity scores, showed close discrimination to APACHE-IV and SAPS-II for predicting in-hospital and long-term mortality.
Baseline ICH volume, hematoma expansion, infratentorial/deep location, and ventricular extension were shown to be independent predictors of poor prognosis and mortality of ICH [30]. It was found in a previous study that common intensive care scoring systems (including SAPS-II) did not show superior performance in 6-month mortality in comparison with a simpler model based on GCS and age [27]. In another study, both SAPS-II and GCS were found to be good predictors of mortality [31]. In the present study, variables that have been shown to have a substantial role in the outcomes of patients with ICH were also evaluated. It was found that one-year mortality rate was higher in patients who have lower GCS on the first admission and who become severely hypotensive requiring vasopressors (regardless of blood pressure-lowering therapy) within the first 24 h of admission. Further analysis showed that the need for mechanical ventilation, hematoma volume, the presence of intraventricular hemorrhage, and hematoma originating from lobar and nonlobar regions together were found to be significant prognosticators of one-year mortality in patients with ICH.
Neurosurgery (hematoma evacuation and/or EVD insertion) was observed to significantly reduce mortality in supraventricular hematomas [32]. In the same study, no difference was observed in terms of survival in patients with EVD implantation. In our study, although the mortality rate was less in patients undergoing surgery than those treated only medically, this difference was not statistically significant. However, the mortality rate was significantly increased in patients with EVD.
Although one-month mortality in ICH was reported as 40%, one-year mortality can be up to 54.7% [7, 33]. The calculated in-hospital mortality rate of this study was 42.3%. This number may seem to be relatively high; however, there are some issues that may have contributed to this result. In some studies, patients who received palliative treatment in ICUs and underwent early care limitations considering that the further treatment would be futile were not included in mortality calculations [11]. According to Turkish Criminal Law, withdrawal of life support and do-not-resuscitate orders are considered as passive euthanasia by jurists, so none of them are used [34]. Additionally, because of the lack of adequate palliative care centers in the country, the length of ICU stay of some patients is prolonged unnecessarily (ranging from 2 to 87 days in this study). Among the in-hospital deceased group, 22 patients stayed in the ICU for more than 20 days. This fact may contribute to a rise in the in-hospital mortality rates because they cannot be discharged from the ICU after the end of their intensive therapy. According to the results, 21 patients died in ICU because of hospital-acquired infections, and the mean hospital stay time of these patients was 38.9 ± 22.7 days. Moreover, nine patients had been declared brain dead, and eight patients died because of the medical causes related to initial brain damage (Supplemental Table). As in-hospital mortality is associated with the social insurance policy of the country, religiosity, ethnicity, hospital characteristics, discharge destinations (hospice, palliative care, home), end of life decisions, and patient selection bias, it would be appropriate to evaluate the mortality studies by considering these variables [35, 36].
The ICH score was the first developed grading scale for patients with ICH and is still widely used in many institutions. Later, several ICH-specific, neurologic evaluation, and radiology-based prognostic scales (including max-ICH, Essen-ICH, and ICH-FOS) were created and tested for prognostication performance over the original ICH grading scale [5–10]. There are very few studies comparing simple ICH-specific grading scales and routine ICU scoring systems. The original ICH score was found to have better discrimination for one-month mortality in patients with ICH [15]. A possible reason for this result could be that the authors excluded patients with comorbidities, such as renal failure or ischemic heart disease, and patients who necessitated neurosurgical interventions for ICH [15]. Under these circumstances, it would not be a surprise for APACHE-II to underscore while excluding these parameters. In the present study, although all three ICH-specific grading scales provided satisfactory results for predicting one-year mortality, the common intensive care severity scoring systems showed better performance.
Numerous factors affect the prognosis of patients with ICH. Although intensive care scoring systems contain many related prognostic factors, they are not simple and practical, so that the use of a calculator is needed. On the other hand, since simple grading scales ignore many factors that affect prognosis, they cannot be sufficient to guide treatment. In an ideal scoring system, a balance should be established between simplicity and predictability [37]. Therefore, none of these scoring systems are perfect.
There are several limitations of the study. First is that the scores were calculated retrospectively relying on the medical records. Second, although all these scores were calculated by a single researcher using the same calculator, when it comes to daily practice, bias due to differences in calculations among medical personnel cannot be excluded entirely. Therefore, these scores should not be used for decision-making, and the interpretation of this study needs to be used cautiously in clinical practice. Another limitation is that the study is monocentric and had a small sample size in comparison with nationwide studies; hence, it may not generally be applicable to a larger population. Although long-term mortality rates were evaluated, this study did not include the causes of death at the end of the first year and long-term functional outcomes. All causes of in-hospital death may have been affected by the previously mentioned reasons leading to prolonging hospital stay. Therefore, different from the previous studies, the most common cause of in-hospital mortality of ICH patients in this study is infections and infection-related situations (sepsis, MODS, etc.). Additionally, institutional and personal differences in management planning, including the decision for surgery, directing medical and pharmacological treatment, transferring patients to wards, stroke units, ICU, or neuro-ICU, should be considered.
Conclusions
The need for mechanical ventilation, hematoma volume, the presence of intraventricular hemorrhage, and hematoma originating from both lobar and nonlobar regions were strong independent prognosticators of one-year mortality in patients with ICH.
Although all three ICH-specific grading scales (ICH, max-ICH, Essen-ICH, and ICH-FOS) provided satisfactory results for predicting one-year mortality, the common intensive care severity scoring systems (LODS, SAPS-II, SAPS-III-ICU, and APACHE-IV) showed better performance. Intensive care severity scoring systems evaluate patients with ICH multi-systemically; therefore, it could be suggested that these calculations would preferably be used for estimating the prognosis of patients treated in ICUs, and neurologic evaluation and radiology-based ICH-specific grading scales could be used as a communication tool.
Considering the possibility that patients with ICH can rapidly deteriorate within hours, the SAPS-III score may be recommended for use in EDs for triage after proper customization. However, the interpretation of this result needs to be used cautiously in clinical practice, and further studies are needed to validate SAPS-III before it can be used in EDs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author Contribution
The manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. I further confirm that the order of authors listed in the manuscript has been approved by all of us.
Source of Support
No funding received for this study.
Conflicts of interest
All authors declare that they have no conflicts of interest.
Ethical Approval
This study was conducted in adherence to ethical guidelines after receiving ethical approval (Derince Training and Research Hospital Clinical Trials Ethical Board, Decree: 2019:51).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Steiner T, Petersson J, Al-Shahi Salman R, et al. European Research Network on Intracerebral Haemorrhage. European research priorities for intracerebral haemorrhage. Cerebrovasc Dis. 2011;32:409–419. doi: 10.1159/000330653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Asch CJ, Luitse MJ, Rinkel GJ, Van Der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 4.Hemphill JC, Greenberg SM, Anderson CS, American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 5.Hemphill JC, III, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.STR.32.4.891. [DOI] [PubMed] [Google Scholar]
- 6.Sembill JA, Gerner ST, Volbers B, et al. Severity assessment in maximally treated ICH patients: the max-ICH score. Neurology. 2017;89:423–431. doi: 10.1212/WNL.0000000000004174. [DOI] [PubMed] [Google Scholar]
- 7.Ji R, Shen H, Pan Y, et al. A novel risk score to predict 1-year functional outcome after intracerebral hemorrhage and comparison with existing scores. Crit Care. 2013;17:R275. doi: 10.1186/cc13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weimar C, Benemann J, Diener HC. Development and validation of the Essen intracerebral haemorrhage score. J Neurol Neurosurg Psychiatry. 2006;77:601–605. doi: 10.1136/jnnp.2005.081117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satopää J, Mustanoja S, Meretoja A, et al. Comparison of all 19 published prognostic scores for intracerebral hemorrhage. J Neurol Sci. 2017;379:103–108. doi: 10.1016/j.jns.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 10.Suo Y, Chen WQ, Pan YS, et al. The max-intracerebral hemorrhage score predicts long-term outcome of intracerebral hemorrhage. CNS Neurosci Ther. 2018;24:1149–1155. doi: 10.1111/cns.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt FA, Liotta EM, Prabhakaran S, Naidech AM, Maas MB. Assessment and comparison of the max-ICH score and ICH score by external validation. Neurology. 2018;91:939–946. doi: 10.1212/WNL.0000000000006117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med. 2006;34:1297–1310. doi: 10.1097/01.CCM.0000215112.84523.F0. [DOI] [PubMed] [Google Scholar]
- 13.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1994;271:1321. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 14.Le Gall JR, Klar J, Lemeshow S, et al. The logistic organ dysfunction system: a new way to assess organ dysfunction in the intensive care unit. JAMA. 1996;276:802–810. doi: 10.1001/jama.1996.03540100046027. [DOI] [PubMed] [Google Scholar]
- 15.Pan K, Panwar A, Roy U, Das BK. A comparison of the intracerebral hemorrhage score and the acute physiology and chronic health evaluation II score for 30-day mortality prediction in spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2017;26:2563–2569. doi: 10.1016/j.jstrokecerebrovasdis.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Republic of Turkey Ministry of Health (2012) Public Health Agency of Turkey death registry system circular (in Turkish). https://www.saglik.gov.tr/TR,11167/olum-bildirim-sistemiobs-genelgesi.html.
- 17.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27:1304–1305. doi: 10.1161/01.STR.27.8.1304. [DOI] [PubMed] [Google Scholar]
- 18.Hallevi H, Dar NS, Barreto AD, et al. The IVH score: a novel tool for estimating intraventricular hemorrhage volume: clinical and research implications. Crit Care Med. 2009;37:969–974. doi: 10.1097/CCM.0b013e318198683a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falcone GJ, Biffi A, Brouwers HB, et al. Predictors of hematoma volume in deep and lobar supratentorial intracerebral hemorrhage. JAMA Neurol. 2013;70:988–994. doi: 10.1001/jamaneurol.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter JV, Pan J, Rai SN, Galandiuk S. ROC-ing along: evaluation and interpretation of receiver operating characteristic curves. Surgery. 2016;159:1638–1645. doi: 10.1016/j.surg.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 21.Karabıyık L. Intensive care scoring systems. J Med Surg Intensive Care Med. 2010;9:129–143. [Google Scholar]
- 22.Steiner T, Al-Shahi Salman R, Beer R, European Stroke Organisation et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9:840–855. doi: 10.1111/ijs.12309. [DOI] [PubMed] [Google Scholar]
- 23.Ghorbani M, Ghaem H, Rezaianzadeh A, Shayan Z, Zand F, Nikandish R. A study on the efficacy of APACHE-IV for predicting mortality and length of stay in an intensive care unit in Iran. F1000Research. 2017;6:2032. doi: 10.12688/f1000research.12290.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayazoglu TA. Validation of the APACHE IV scoring system in patients with stroke: a comparison with the APACHE II system. Anaesth Pain Intensive Care. 2011;15:7–12. [Google Scholar]
- 25.Huang Y, Chen J, Zhong S, Yuan J. Role of APACHE II scoring system in the prediction of severity and outcome of acute intracerebral hemorrhage. Int J Neurosci. 2016;126:1020–1024. doi: 10.3109/00207454.2015.1099099. [DOI] [PubMed] [Google Scholar]
- 26.Moon BH, Park SK, Jang DK, Jang KS, Kim JT, Han YM. Use of APACHE II and SAPS II to predict mortality for hemorrhagic and ischemic stroke patients. J Clin Neurosci. 2015;22:111–115. doi: 10.1016/j.jocn.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Fallenius M, Skrifvars MB, Reinikainen M, Bendel S, Raj R. Common intensive care scoring systems do not outperform age and glasgow coma scale score in predicting mid-term mortality in patients with spontaneous intracerebral hemorrhage treated in the intensive care unit. Scand J Trauma Resusc Emerg Med. 2017;25:102. doi: 10.1186/s13049-017-0448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metnitz PG, Moreno RP, Almeida E, et al. SAPS 3—from evaluation of the patient to evaluation of the intensive care unit. Part 1: objectives, methods and cohort description. Intensive Care Med. 2005;31:1336–1344. doi: 10.1007/s00134-005-2762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakr Y, Krauss C, Amaral AC, et al. Comparison of the performance of SAPS II, SAPS 3, APACHE II, and their customized prognostic models in a surgical intensive care unit. Br J Anaesth. 2008;101:798–803. doi: 10.1093/bja/aen291. [DOI] [PubMed] [Google Scholar]
- 30.Esfahani DR, Radnis CA, Hussein AE, Amin-Hanjani S, Charbel FT, Alaraj A. Thresholds for volume and expansion in intraparenchymal hemorrhage: predictors of neurologic deterioration and mortality. World Neurosurg. 2017;106:131–138. doi: 10.1016/j.wneu.2017.06.131. [DOI] [PubMed] [Google Scholar]
- 31.Handschu R, Haslbeck M, Hartmann A, et al. Mortality prediction in critical care for acute stroke: severity of illness-score or coma-scale? J Neurol. 2005;252:1249–1254. doi: 10.1007/s00415-005-0853-5. [DOI] [PubMed] [Google Scholar]
- 32.Rivera-Fernández R, Guerrero-López F, Rodríguez-Rubio D, et al. Survival analysis of surgically evacuated supratentorial spontaneous intracerebral hemorrhage with intraventricular extension. Neurocirugia (Astur) 2016;27:220–228. doi: 10.1016/j.neucir.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:660–667. doi: 10.1136/jnnp-2013-306476. [DOI] [PubMed] [Google Scholar]
- 34.Demir M. End-of-life decisions and their legal status in Turkey. Acta Medica. 2014;3:42–45. [Google Scholar]
- 35.Naidech AM, Bernstein RA, Bassin SL, et al. How patients die after intracerebral hemorrhage. Neurocrit Care. 2009;11:45–49. doi: 10.1007/s12028-009-9186-z. [DOI] [PubMed] [Google Scholar]
- 36.Ormseth CH, Falcone GJ, Jasak SD, et al. Minority patients are less likely to undergo withdrawal of care after spontaneous intracerebral hemorrhage. Neurocrit Care. 2018;29:419–425. doi: 10.1007/s12028-018-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang BY, Appelboom G, Kellner CP, et al. Clinical grading scales in intracerebral hemorrhage. Neurocrit Care. 2010;13:141–151. doi: 10.1007/s12028-010-9382-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.