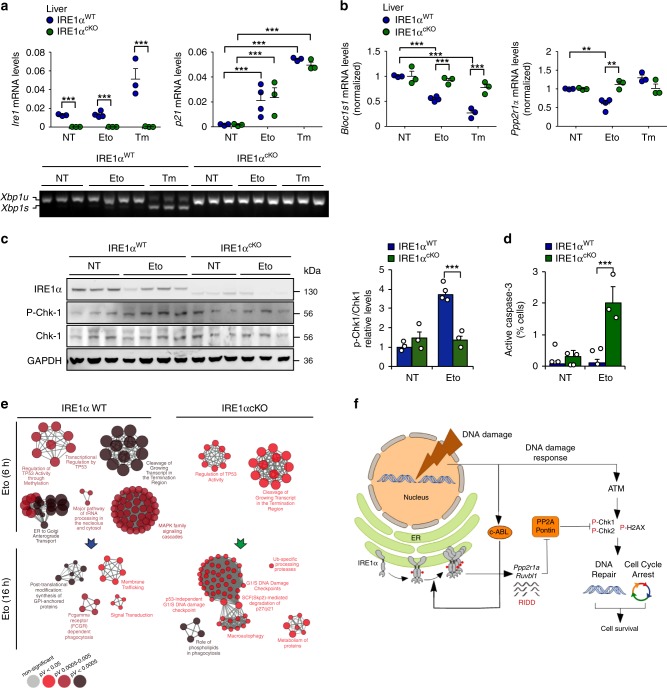
Fig. 6. IRE1α deletion in liver alters the DDR under genotoxic stress.
a IRE1α was conditionally deleted the liver using the MxCre and LoxP system (IRE1αcKO). Mice were intraperitoneally injected with 50 mg/Kg etoposide (Eto) or 1 mg/Kg tunicamycin (Tm) and sacrificed 6 h and 16 h later. Total mRNA levels of the deleted IRE1, and p21 were measured 6 h later in the liver by real-time-PCR (n = 3–4 mice per group). Xbp1 mRNA splicing (bottom panel) was monitored in the same samples by RT-PCR. b Liver extracts of animals described in a, Ppp2r1a and Bloc1s1 mRNA expression levels were measured 6 h later of Eto treatment by real-time-PCR (n = 3). c Protein liver extracts were obtained from mice treated described in a and the expression levels of indicated proteins were monitored 6 h later of Eto treatment by western blot. Quantification of the levels of p-CHK1 is shown (Right panel). d Mice from a were intraperitoneally injected with 50 mg/Kg Eto and sacrificed 48 h later. Then, livers active-caspase 3 detected by immunohistochemistry (n = 2–3). e Gene expression profile analysis was performed in mRNA from liver extracts of animals described in a. Most significant pathways altered by Eto administration in WT and IRE1α null livers are shown. Three independent biological samples were used. In all panels, data is shown as mean ± s.e.m.; *p < 0.05, **p < 0.01, and ***p < 0.001, based on a–d two-way ANOVA followed Bonferroni’s test. Data is provided as a Source Data file. f Working model: genotoxic stress activates IRE1α in the absence of ER stress markers, selectively engaging RIDD. IRE1α degrades mRNAs involved in the DNA damage response encoding for Ppp2r1a and Ruvbl1, regulating the (de)phosphorylation of the histone H2AX and CHK-1/2. The non-canonical activation of IRE1α involves the participation of the c-Abl kinase that is activated by DNA damage response kinases as ATM. The expression and function of IRE1α is essential to promote survival under DNA damage conditions by controlling cell cycle arrest and DNA repair programs.