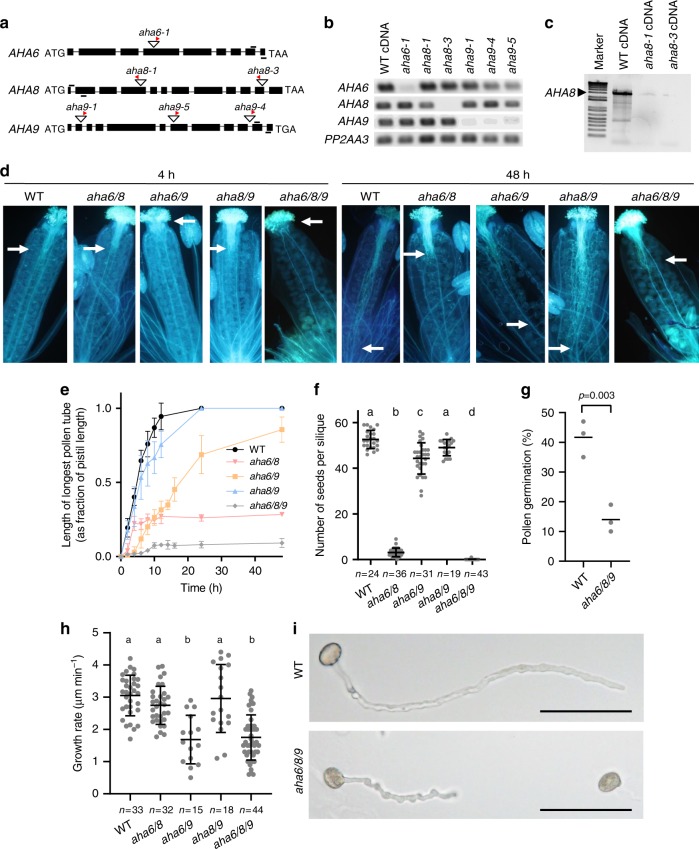
Fig. 2. AHA double and triple mutants display aberrant pollen tube phenotypes.
a Diagram of gene structures with position of T-DNA insertions (triangles) in the genes. Red arrowheads indicate the T-DNA left border. Exons are indicated as black rectangles, and introns as black lines. Primers used for reverse-transcription PCR (RT-PCR) are indicated by short black lines above the gene representations. b RT-PCR analysis of T-DNA insertion lines using primer pairs indicated in (a). RNA was from flowers at stage 1271. PP2AA3 served as a positive control. c RT-PCR analysis of T-DNA insertion lines using primers that amplify full-length cDNA of AHA8. d Aniline blue-stained pollen tubes of ms1 pistils pollinated with different aha mutant lines. White arrows indicate the position of the longest tubes at 48 h after pollination. e In vivo pollen tube growth rate (as a fraction of pollen tube length over pistil length), as revealed by aniline blue staining (n ≥ 3 for all genotypes and time points; error bars show SD). f Number of seeds per silique (one-way ANOVA and Bonferroni’s Multiple Comparison Test; p < 0.01; error bars show mean with SD). g In vitro germination rate for WT and aha6/8/9 triple mutant pollen. Means from three individual experiments with ≥ 100 scored pollen grains for each genotype and replicate are shown (two-sided t-test; p < 0.003). h Average pollen tube growth rate assayed in vitro (one-way ANOVA; Bonferroni’s test; p < 0.01; error bars show mean with SD). i Light microscopy images of WT and aha6/8/9 pollen tubes grown in vivo. The triple mutant is characterized by short and wavy pollen tubes. Scale bar = 100 µm.