Figure 2. Treg depletion results in pancreatitis and promotes PanIN formation and progression.
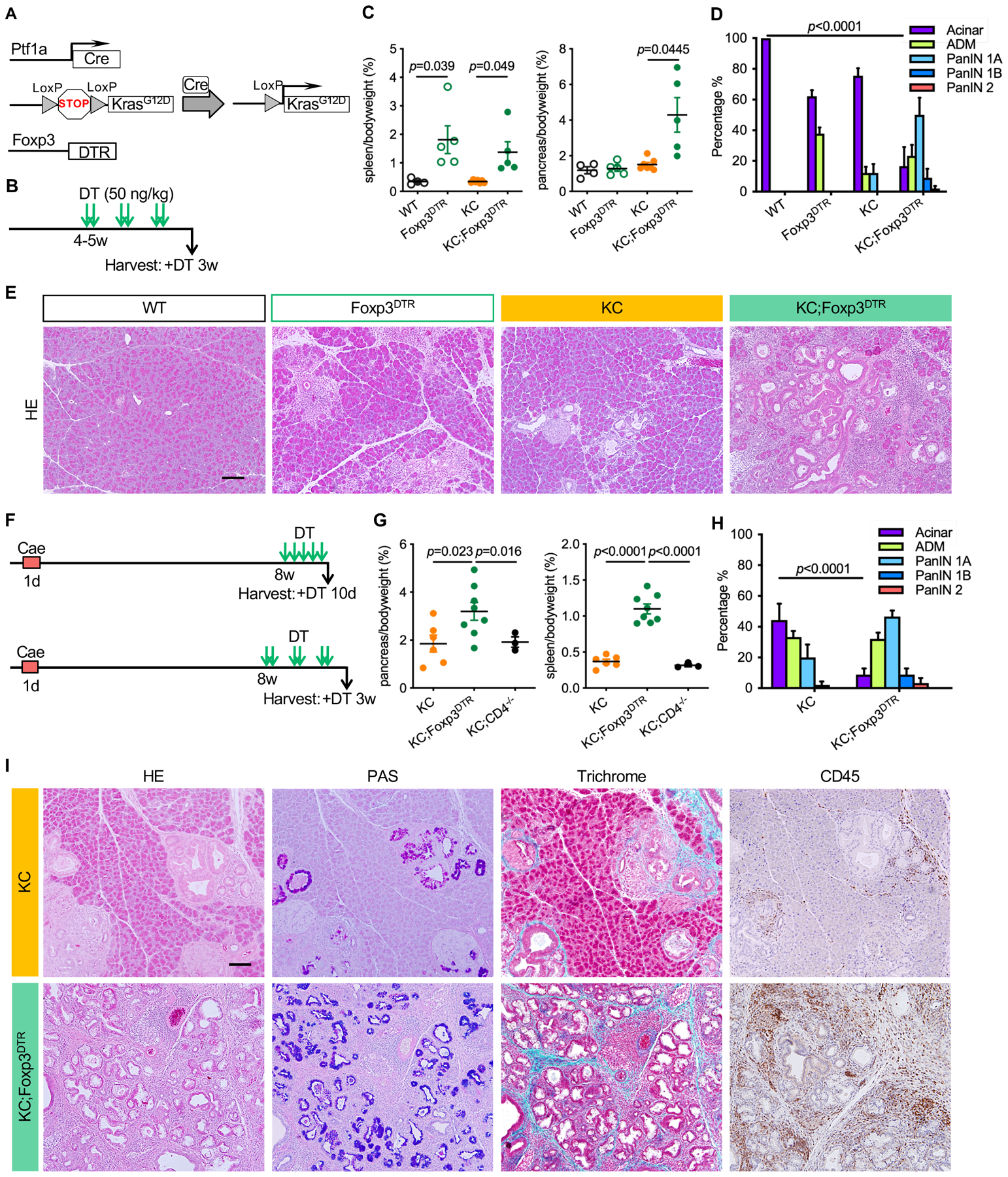
(A) Genetic makeup of the KC;Foxp3DTR mouse model. (B) Experimental design, n=4–7mice/cohort. (C) Pancreas to body weight ratio and spleen to body weight ratio of WT, Foxp3DTR, KC and KC;Foxp3DTR mice after 3 weeks of DT treatment. Data represent mean ± SEM, n=4–7mice/cohort. The statistical difference was determined by two-tailed t-tests. (D) Histopathologic quantification of WT, Foxp3DTR, KC and KC;Foxp3DTR mice after 3 weeks of DT treatment. Data represent mean ± SEM, n = 3–4 mice/cohort. The statistical difference was determined by two-way ANOVA. (E) H&E staining of WT, Foxp3DTR, KC and KC;Foxp3DTR pancreata after 3 weeks of DT treatment. Scale bar 100 μm. (F) Experimental design, n=3–8 mice/cohort. (G) Pancreas to body weight ratio and spleen to body weight ratio of KC, KC;Foxp3DTR and KC;CD4−/− mice that received 3 weeks of DT treatment starting 8 weeks post caerulein. Data represent mean ± SEM, n=3–8 mice/cohort. The statistical difference was determined by two-tailed t-tests. (H) Histopathologic quantification of KC and KC;Foxp3DTR mice received 3 weeks of DT treatment starting 8 weeks post caerulein.. Data represent mean ± SEM, n=3–4mice/cohort. The statistical difference was determined by two-way ANOVA. (I) H&E staining, Periodic acid–Schiff (PAS) staining, Gomori trichrome staining and immunohistochemistry staining for CD45 in KC and KC;Foxp3DTR mice that received 3 weeks of DT treatment starting 8 weeks post caerulein. Scale bar 100 μm.
