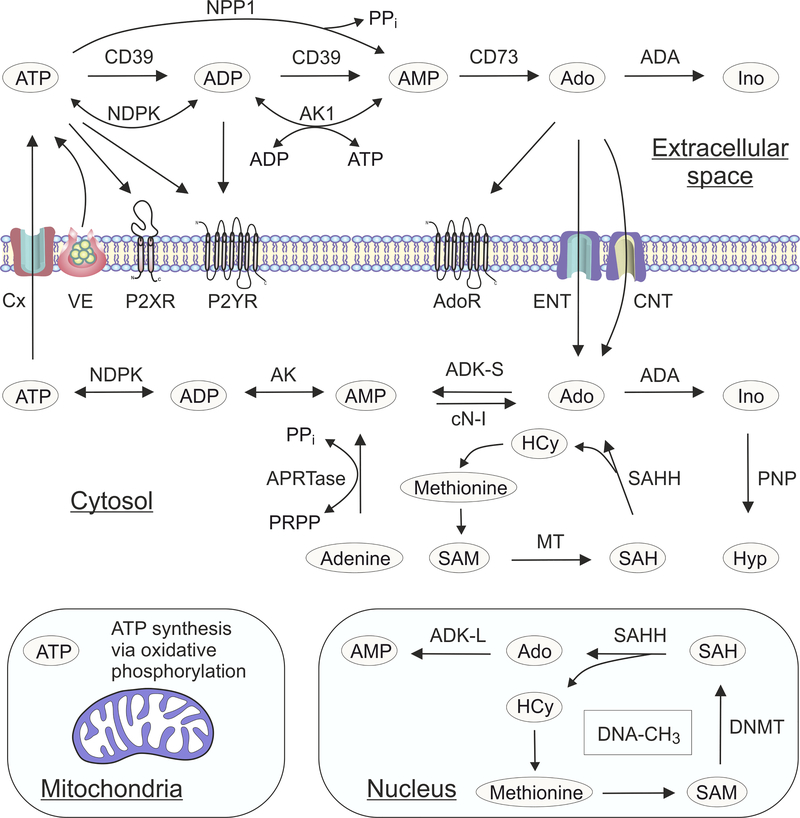
Figure 1. Compartmentalization of adenosine biochemistry.
Extracellular purine turnover is comprised of the release of endogenous ATP upon cell damage or via non-lytic mechanisms of vesicular exocytosis (VE), connexin hemichannels (Cx) and other ion channels and transporters; the triggering of signaling events via nucleotide (P2XR and P2YR) and adenosine (AdoR) selective receptors; the metabolism of nucleotides and nucleosides; and the uptake of nucleotide-derived adenosine via equilibrative (ENT) and concentrative (CNT) nucleoside transporters. General schemes of adenosine producing and removing pathways have included a role for the enzymes nucleoside triphosphate diphosphohydrolase-1 (NTPDase1/CD39), nucleotide pyrophosphatase/ phosphodiesterase-1 (NPP1), ecto-5′-nucleotidase/CD73 and adenosine deaminase (ADA). In addition, counteracting adenylate kinase-1 (AK1) and nucleotide diphosphokinase (NDPK) activities contribute to the regeneration of extracellular ATP via reversible phosphotransfer reactions. Intracellular adenosine metabolism depends on the cytoplasmic form of adenosine kinase (ADK-S), and the enzymes ADA, cytosolic nucleotidase (cN-I), purine nucleoside phosphorylase (PNP), and adenine phosphoribosyl transferase (APRTase). S-Adenosylhomocysteine (SAH) can also be hydrolyzed to adenosine (Ado) and homocysteine (HCy) by SAH hydrolase (SAHH), while S-adenosylmethionine (SAM) serves as the donor of a methyl group in the transmethylation reactions catalyzed by methyltransferases (MT). Because mitochondria are the main source of ATP production, mitochondrial bioenergetics is tightly linked to adenosine homeostasis. In the cell nucleus adenosine is part of the transmethylation pathway, which adds methyl groups to DNA (DNA-CH3) with DNA methyltransferase (DNMT). The nuclear form of adenosine kinase (ADK-L) drives the flux of methyl groups through the pathway leading to increased DNA and histone methylation. For the sake of clarity only the most important enzymes are mentioned.