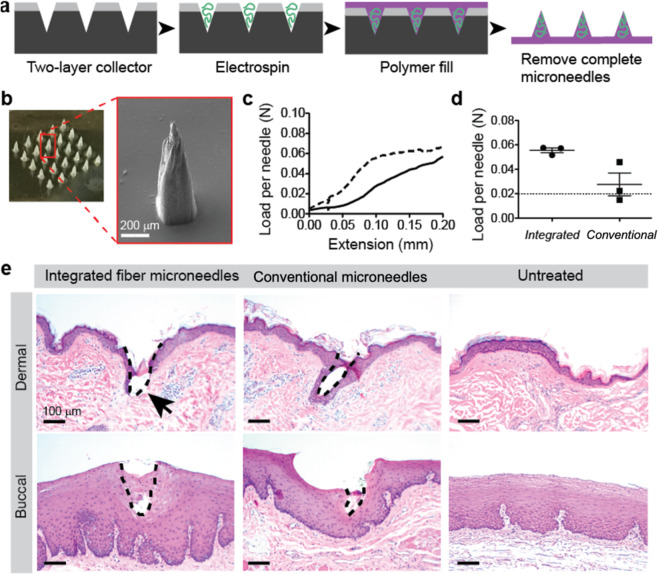
Figure 6.
Fabrication of mechanically robust integrated fiber microneedles. (a) Integrated fiber microneedle fabrication approach. (b) Optical microscope image of a completed integrated fiber microneedle array and (inset) SEM image of a single integrated fiber microneedle. (c) Graph of load and extension per needle for compression of 3 × 3 microneedle arrays between two steel plates using an Instron universal testing system. Curve represents the mean of three integrated fiber microneedle arrays (dashed line) and conventional matrix microneedles (solid line) from separate PDMS-based collectors. Error bars omitted for graph clarity. (d) Failure forces per needle for integrated fiber microneedles compared to conventional matrix microneedles. For all samples measured with this method, the failure force was taken as the load at 0.1 mm extension (n = 3, error bars represent standard deviations). (e) Optical microscopy of dermal and buccal tissue treated with conventional matrix microneedles or integrated fiber microneedles demonstrating integrated fiber microneedle disruption of the dermal stratum corneum and viable epidermis and conventional matrix microneedle and integrated fiber microneedle access to the epithelium of buccal tissue. Untreated dermal and buccal tissue controls are provided to demonstrate the native structure of both tissues.