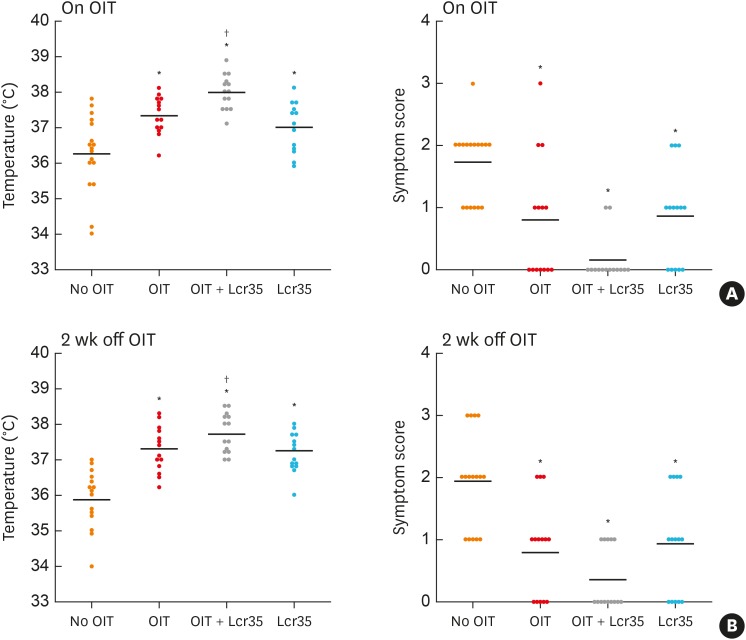
Fig. 2. Anaphylaxis symptom scores and rectal temperature. Mice were orally challenged with ovomucoid on day 1 and 14 after discontinuation of OIT and/or Lcr35 treatment. (A) Day 1 after discontinuation of OIT (on OIT) and (B) day 14 after discontinuation of OIT.
OIT, oral immunotherapy.
*P < 0.05 compared to the control group (vs. No OIT); †P < 0.05 compared to the OIT group (vs. OIT).