Abstract
Aims
Several signaling pathways contribute to endothelial-mesenchymal transitions and vascular calcification, including bone morphogenetic protein (BMP) and transforming growth factor (TGF) β signaling. The transcription factor homeobox D3 (Hoxd3) is known to regulate an invasive endothelial phenotype, and the aim of the study is to determine if HOXD3 modulates BMP and TGFβ signaling in endothelium.
Methods and Research
We report that endothelium with high BMP activity due to the loss of BMP inhibitor matrix Gla protein (MGP) shows induction of Hoxd3. HOXD3 is part of a BMP-triggered cascade. When activated by BMP9, ALK1 induces HOXD3 expression. Hoxd3 promoter is a direct target of phosphorylated (p) SMAD1, a mediator of BMP signaling. High BMP activity further results in enhanced TGFβ signaling due to induction of TGFβ1 and its receptor, ALK5. This is mediated by HOXD3, which directly targets the Tgfb1 promoter. Finally, TGFβ1 and BMP9 stimulate the expression of MGP, which limits the enhanced ALK1 induction by counteracting BMP4. The cascade of BMP9-HOXD3-TGFβ also affects Notch signaling and angiogenesis through induction of Notch ligand Jagged 2 and suppression of Notch ligand Delta-like 4 (Dll4).
Conclusion
The results suggest that HOXD3 is a novel link between BMP9/ALK1 and TGFβ1/ALK5 signaling.
Translational Perspective
BMP and TGFβ signaling are instrumental in vascular disease such as vascular calcification and atherosclerosis. This study demonstrated a novel type of crosstalk between endothelial BMP and TGFβ signaling as mediated by HOXD3. The results provide a possible therapeutic approach to control dysfunctional BMP and TGFβ signaling by regulating HOXD3.
Keywords: Endothelium, Bone morphogenetic protein, Notch, Activin receptor-like kinase 1, Vascular development
Introduction
HOXD3 is part of the HOXD gene family and plays an important role in developmental processes, such as the formation of somatic mesoderm and neural crest [1, 2]. HOXD3 is also involved in the conversion of the endothelium from quiescence to the angiogenic state [3–5]. Interestingly, when targeted by microRNA miR-203a, HOXD3 can also suppress angiogenesis and cell metastasis in hepatocellular carcinoma through the VEGF receptor [6]. The HOXD3 expression is associated with a positional identity in endothelial cells (ECs), exhibiting high levels of HOXD3 in venous ECs, and moderate levels in arterial ECs [7].
The BMPs are members of the transforming growth factor beta (TGFβ) superfamily and are critical for angiogenesis in embryos as well as vascular homeostasis in adults [8–10]. The BMP and TGFβ ligands elicit their activity through the activation of receptor complexes composed of type I and type II Ser/Thr kinase receptors. Seven type I receptors, termed activin receptor-like kinase (ALK1 to ALK7), determine the specificity of BMP signal in concert with five type II receptors [8]. When BMP or TGFβ bind the receptor complex, the type II receptor activates the type I receptor, which typically propagates the signaling by phosphorylating SMADs. BMP signaling preferentially activate receptor-activated (R)-SMAD1, 5 and 8, while TGFβ signaling phosphorylates SMAD 2 and 3 [11–13]. The activity of the BMPs is modulated by a number of extracellular antagonists such as matrix Gla protein (MGP) [14], which is highly expressed in the endothelium where it primarily regulates BMP4 [15].
Here, we show that the lack of MGP increases the expression of HOXD3 through upregulation of the BMP9/ALK1 pathway. The absence of MGP allows BMP4 to induce ALK1 [16]. HOXD3 subsequently binds to the promoter of TGFβ1 and triggers its expression, resulting in regulatory effects on MGP and Notch signaling. Together, the study suggests that HOXD3 is a novel link between BMP and TGFβ signaling.
Results
BMP activation induces HOXD3 in the vascular endothelium
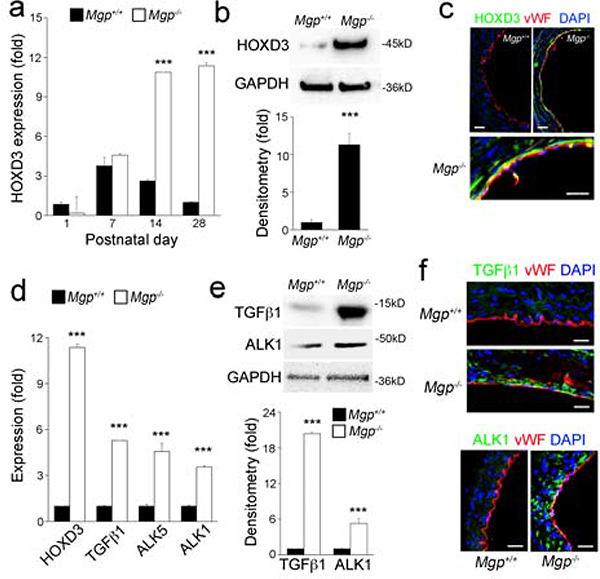
To assess the relationship between BMP activation and HOXD3 in the vasculature, we examined the expression of HOXD3 in Mgp−/− aortas on postnatal day (P) 1 to 28, when BMP signaling is highly activated [17–19]. The results showed a significant induction of HOXD3 starting on P14 as determined by real-time PCR (Figure 1a). Similar levels of HOXD3 expression were maintained through P28 (Figure 1a–b), when most of the Mgp−/− mice died due to vascular abnormalities [16, 20]. Immunostaining showed that HOXD3 co-localized with the endothelial marker von Willebrand factor (vWF) (Figure 1c), suggesting that the endothelium is responsible for the HOXD3 increase. We also found that the induction of HOXD3 was accompanied by enhanced expression of TGFβ1, ALK1 and ALK5, as detected by real-time PCR, immunoblotting and immunostaining (Figure 1d–f). This points to the possibility that BMP-induced HOXD3 is involved in TGFβ signaling.
Figure 1. Lack of MGP induces HOXD3 expression and TGFβ / BMP signaling in aortic tissue.
(a-c) Expression of HOXD3 in Mgp−/− aortic tissue examined between postnatal day (P) 1 to 28 by real-time PCR (a), immunoblotting with densitometric analysis (b) and immunostaining on P28 (c) (n=6).
(d-e) Expression of HOXD3, TGFβ1, ALK5, ALK1 in Mgp−/− aortic tissue was examined on P28 by real-time PCR (d) and immunoblotting with densitometric analysis (e).
(f) Immunostaining of ALK1 and TGFβ1 in Mgp−/− aortic tissue on P28.
Data were analyzed by Student’s t test. ***, P<0.001. Error bars are standard deviation. ***, P < 0.001. Scale bar, 50 μm.
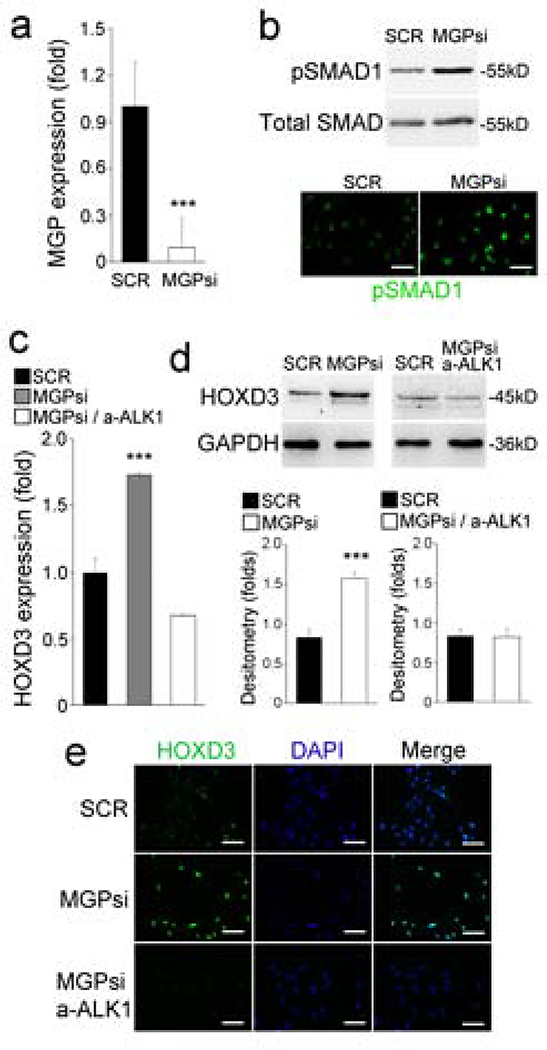
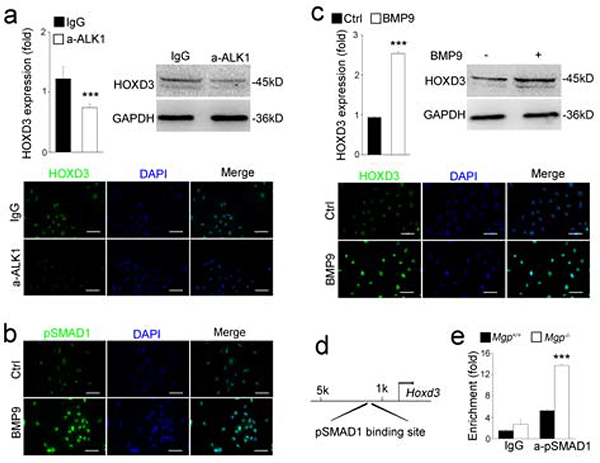
To investigate how BMP signaling induced HOXD3 expression, we performed three sets of experiments. First, we depleted MGP in HUVECs by using lentiviral vectors carrying MGP siRNA. The depletion was confirmed by a reduction in MGP expression as detected by real-time PCR (Figure 2a) and increased levels of pSMAD1, as detected by immunoblotting and immunostaining (Figure 2b). Real-time PCR, immunoblotting and immunostaining all showed that the depletion of MGP induced HOXD3 (Figure 2c–e). Since previous studies showed that BMP9/ALK1 is highly induced in MGP-deficiency [21], we next inhibited BMP9 activity in the MGP-depleted HUVECs by using anti-ALK1 neutralizing antibodies (10 ug/ml). As expected, we found that suppression of BMP9/ALK1 signaling abolished the induction of HOXD3 (Figure 3a and c), suggesting that BMP9/ALK1 signaling mediated the HOXD3 induction. Finally, we treated the HUVECs with BMP9 (10 ng/ml) for 24 hours, and confirmed BMP activation by immunostaining for pSMAD1 (Figure 3b). We found that BMP9 induced the expression of HOXD3 as determined by real-time-PCR, immunoblotting and immunostaining (Figure 3c).
Figure 2. Depletion of MGP increases HOXD3 expression in HUVECs.
(a) Expression of MGP in HUVECs transfected with MGP siRNA. SCR, scrambled siRNA.
(b) Levels of pSMAD1 detected by immunoblotting and immunostaining.
(c-e) HOXD3 expression in HUVECs transfected with MGP siRNA and treated with anti-ALK1 antibodies, as shown by real-time PCR (c), immunoblotting with densitometric analysis (d), and immunostaining (e).
Data were analyzed by Student’s t test. ***, P<0.001. Error bars are standard deviation. ***, P < 0.001. Scale bar, 50 μm.
Figure 3. BMP9 induced the expression of HOXD3 through the ALK1 and pSMAD1.
(a) Expression of HOXD3 in HUVECs treated by anti-ALK1 neutralizing antibodies as shown by real-time PCR, immunoblotting and immunofluorescence staining.
(b) Level of pSMAD1 in HUVECs treated with BMP9 as shown by immunostaining.
(c) Induction of HOXD3 in HUVECs treated by BMP9 as shown by real-time PCR, immunoblotting and immunostaining.
(d) pSMAD1 DNA-binding site in the promoter of the Hoxd3 gene.
(e) Increased pSMAD1 DNA-binding at the Hoxd3 promoter in Mgp−/− aortas as shown by ChIP-assays.
Data were analyzed by Student’s t test. ***, P<0.001. Error bars are standard deviation. ***, P < 0.001. Scale bar, 50 μm.
To examine the pSMAD1 DNA-binding site in the promoter of the Hoxd3 gene, we performed ChIP assays using anti-pSMAD1 antibodies to isolate pSMAD1-enriched genomic DNA from Mgp−/− aortas. The results revealed a significant enhancement of pSMAD1 DNA-binding in the promoter of the Hoxd3 gene (Figure 3d–e). Together, the results suggest that BMP9/ALK1 signaling induces HOXD3, and that Hoxd3 is a direct target of SMAD1.
Enhanced HOXD3 induces TGFβ signaling
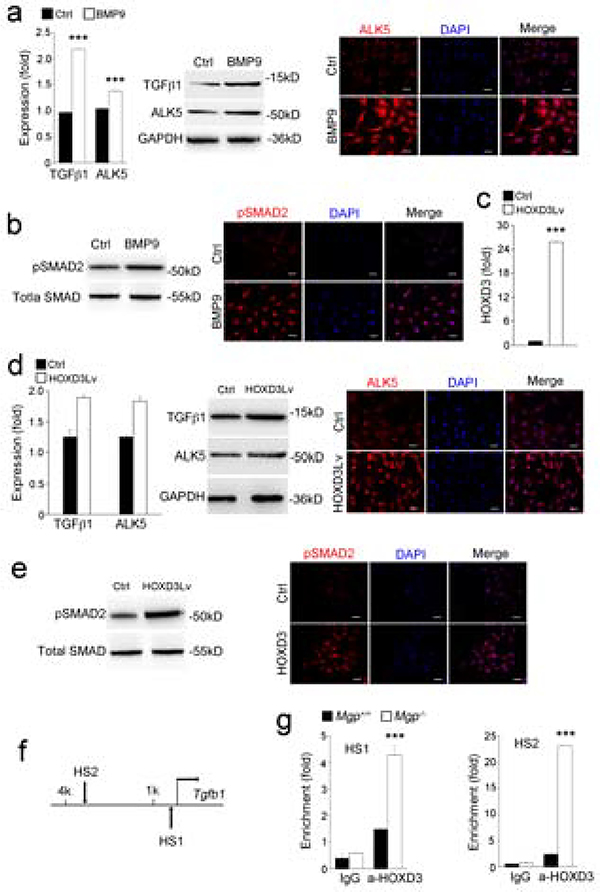
Our results showed that BMP activation induced TGFβ1 and ALK5 in the Mgp−/− aortas (Figure 1). We also found induction of TGFβ1 and ALK5 in BMP9-treated HUVECs, as determined by real-time PCR, immunoblotting and immunostaining (Figure 4a) with a significant increase in pSMAD2/3 (Figure 4b), suggesting that the increased BMP9/ALK1 activity also enhanced TGFβ signaling.
Figure 4. BMP9 induces TGFβsignaling through induction of HOXD3.
(a), Expression of TGFβ1 and ALK5 in HUVECs treated with BMP9 as shown by real-time PCR, immunoblotting and immunostaining.
(b) Activation of pSMAD2 in HUVECs treated with BMP9 as shown by immunoblotting and immunostaining.
(c-d) Expression of HOXD3, TGFβ1 and ALK5 in HUVECs after infection with lentiviral vectors over-expressing HOXD3, as shown by real-time PCR, immunoblotting and immunostaining.
(e) Activation of pSMAD2 in HUVECs after infection with lentiviral vectors over-expressing HOXD3, as shown by immunoblotting and immunostaining.
(f) HOXD3 DNA-binding sites (HS) in the promoter of the Tgfβ1 gene.
(g) Increased HOXD3 DNA-binding in the Tgfβ1 promoter in Mgp−/− aortas as shown by ChIP-assays.
Data were analyzed by Student’s t test. ***, P<0.001. Error bars are standard deviation. ***, P < 0.001. Scale bar, 50 μm.
To investigate if HODX3 mediated the increase in TGFβ signaling, we infected the HUVECs with lentiviral vectors overexpressing HOXD3. The overexpression was confirmed by real-time PCR (Figure 4c). The enhanced HOXD3 induced both the expression of TGFβ1 and ALK5 (Figure 4d) and pSMAD2 signaling (Figure 4e), suggesting that HOXD3 mediates the BMP9 effect on TGFβ signaling.
To determine if TGFβ1 was a direct down-stream target of HOXD3, we examined two putative HOXD3-binding sites in the promoter region of the Tfgb1 gene using ChIP assays (Figure 4f). The results revealed that HOXD3 bound strongly to the two binding sites, which were located around 0.3 and 3 kb upstream of the Tgfb1 gene (Figure 4g). Together our results suggested that the Tgfb1 gene was a direct target of HOXD3.
HOXD3 induces MGP through the induction of TGFβ1, which provides negative feedback regulation for BMP4
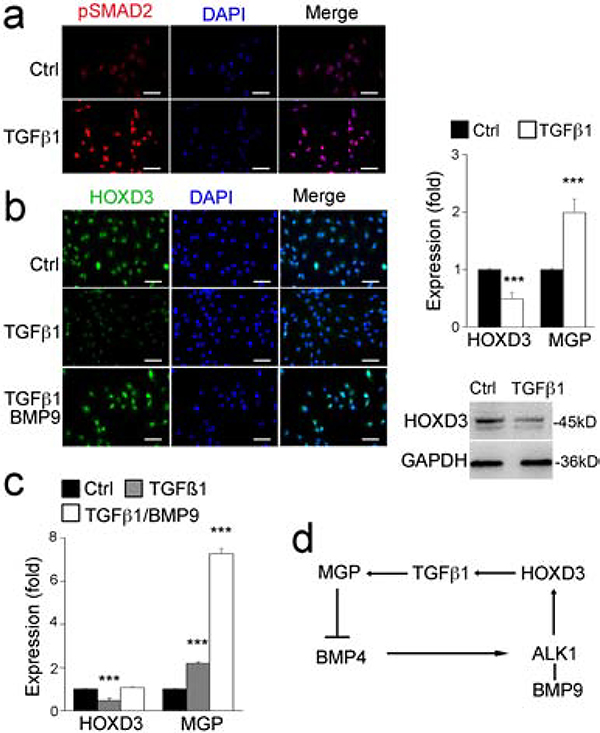
To examine whether HOXD3 provides negative feedback for BMP9/ALK1 signaling, we treated the HUVECs with TGFβ1 (10 ng/ml) for 24 hours. The increased TGFβ1 activity was confirmed by an increase in pSMAD2 immunostaining (Figure 5a). The results showed that the TGFβ1 treatment reduced the expression of HOXD3, but increased MGP expression as shown by real-time PCR and immunoblotting (Figure 5b–c). Interestingly, restoration of BMP9 signaling counteracted the effect of TGFβ1 and normalized HOXD3 expression, but further enhanced MGP expression (Figure 5b–c). Since our previous study showed that ALK1 expression was induced by BMP4 but suppressed by MGP [16], an increase in MGP would suppress ALK1 expression and provide negative feedback for BMP4 and then BMP9/ALK1 signaling (Figure 5f) [22].
Figure 5. TGFβ1 feedback regulates the expression of BMP9, HOXD3 and MGP.
(a) Level of pSMAD2 in HUVECs after treatment with TGFβ1 as shown by immunostaining.
(b-c), Expression of HOXD3 and MGP in HUVECs after treatment with TGFβ1, with or without BMP9, shown by real-time PCR, immunostaining and immunoblotting.
(d) Schematic diagram.
Data were analyzed by Student’s t test. ***, P<0.001. Error bars are standard deviation. ***, P < 0.001. Scale bar, 50 μm.
HOXD3 balances Notch signaling in angiogenesis
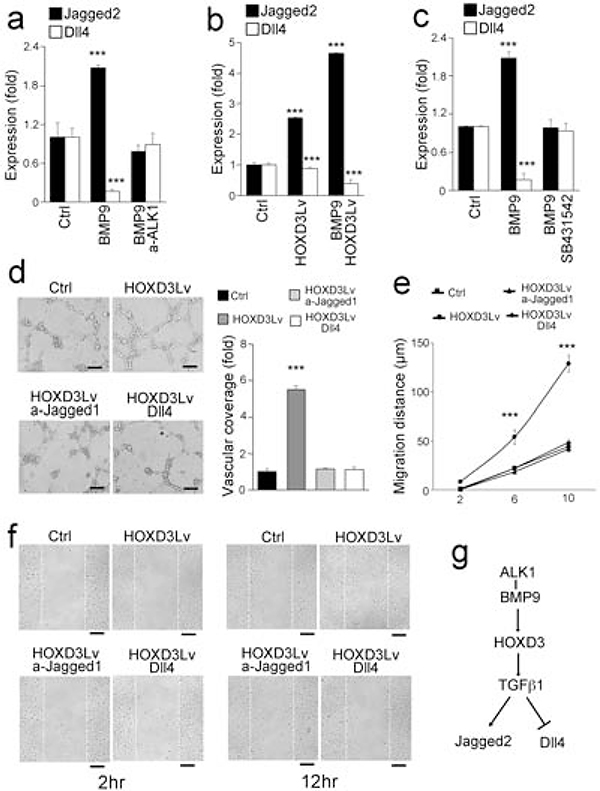
Previous studies have shown that BMP9/ALK1 signaling affects the expression of Notch components [23, 24]. Interestingly, we found that both BMP9 treatment and HOXD3 overexpression affected Notch ligands in HUVECs. The results showed an induction of the Notch ligand Jagged2 and a reduction of the Dll4 ligand, as determined by real-time PCR (Figure 6a–b). These effects were abolished by either anti-ALK1 neutralizing antibodies or the TGFβ inhibitor SB431542, an inhibitor of ALK5 (Figure 5c). It suggests that the crosstalk between BMP9/ALK1 and TGFβ/ALK5 signaling, as mediated by HODX3, also affects the Notch components.
Figure 6. HOXD3 balances BMP9-induced Notch signaling.
(a) Expression of Jagged2 and Dll4 in HUVECs after treatment with BMP9, with or without anti-ALK1 neutralizing antibodies.
(b) Expression of Jagged2 and Dll4 in HUVECs treated with BMP9, with or without infection of lentiviral vectors over-expressing HOXD3.
(c) Expression of Jagged2 and Dll4 in HUVECs after treatment with BMP9, with or without anti-ALK5 neutralizing antibodies.
(d) Angiogenesis assay of HUVECs infected with lentiviral vectors over-expressing HOXD3 and treated with anti-Jagged1 neutralizing antibodies or Dll4 ligands. The vascular coverage was measured and compared between different groups.
(e-f) Migration assay of HUVECs infected with lentiviral vectors over-expressing HOXD3 and treated with anti-Jagged1 neutralizing antibodies or Dll4 ligands.
(g) Schematic diagram.
Data were analyzed by Student’s t test. ***, P<0.001. Error bars are standard deviation. ***, P < 0.001. Scale bar, 50 μm.
To determine if HOXD3-regulated Notch signaling affects angiogenesis, we performed angiogenesis and migration assays using HOXD3-overexpressing HUVECs, which showed a significant increase in the ability to form tubular structures and migrate (Figure 6d–f). We inhibited Jagged2 with neutralizing anti-Jagged2 antibodies or increased the Dll4 activity with excess Dll4 in HOXD3-overexpressing HUVECs. The results showed that either inhibition of Jagged2 or excess Dll4 limited the tube formation and the migratory capacity in the HOXD3-overexpressing HUVECs (Figure 6d–f), suggesting that HOXD3 influences Notch signaling in angiogenesis (Figure 6e).
Discussion
We have identified HOXD3 as a novel link between BMP and TGFβ signaling in the vascular endothelium. We found that lack of MGP allowed for enhanced BMP9/ALK1 signaling to induce HOXD3 through direct action by pSMAD1 on the Hoxd3 promoter, which in turn allowed for HOXD3 to enhance TGFβ1/ALK5 signaling through direct effects on the Tgfb1 promoter. Thus, HOXD3 is in a key position to connect two pathways that are essential in vascular development, and also to affect downstream Notch signaling.
In previous reports, HOXD3 was associated with the conversion from quiescent endothelium to an invasive angiogenic phenotype [3–5], with some similarities to the endothelial-mesenchymal transitions that are known to occur in the Mgp−/− mice [18]. However, it has also been reported that HOXD3 suppresses angiogenesis when targeted by microRNA miR-203a in the setting of hepatocellular carcinoma [6]. Our results add mechanistic details to how HOXD3 may effectuate BMP-stimulated changes in cells responsive to BMP9, through direct promoter effects or through induction of vascular growth factors or inhibitors.
Although our studies were performed in ECs, this system might be active in other vascular cells, such as the smooth muscle cells, which are highly dependent on TGFβ signaling during development [25]. It is also possible that the reported differential expression of HOXD3 in venous versus arterial ECs reflects the balance between BMP and TGFβ signaling in the different vascular beds. Therefore, it is not surprising that the HOXD3 also affected the expression of the Notch ligands Jagged 2 and Dll4, which have been associated with the stalk and tip cell phenotypes, respectively [26], supporting that HOXD3 is involved in the determination of EC fate.
Our previous studies showed that BMP4 is responsible for the induction of ALK1 in ECs through stimulation of ALK2 [27]. The action of BMP4 is counteracted by MGP, which directly binds and inhibits BMP4 as part of negative feedback regulation [15]. However, MGP does not bind BMP9 [28], implying that the suppressive effect of MGP on BMP9/ALK1 signaling is derived from the inhibition of BMP4 and in turn, suppression of ALK1. This is supported by our finding that limiting ALK1 suppressed the expression of HOXD3.
The HUVECs are a unique type of ECs, derived from the umbilical vein, which provides the embryonic liver with blood but receives little or no blood from the fetal liver. Expression of BMP9 has been shown mainly in the liver, which delivers BMP9 to various vascular beds through the circulation [21]. However, it is unclear if BMP9 can cross the placenta, which may limit BMP9 activity in the endothelium of umbilical vein. We found that the HUVECs express BMP9 (Supplemental Figure 1). BMP9 was further induced by lack of MGP and suppressed by TGFβ1 (Supplemental Figure 2), suggesting that BMP9 can also be provided endogenously by the HUVECs.
In summary, we provide novel evidence that BMP9 induces HOXD3 expression to mediate and regulate BMP9/ALK1 signaling, and to provide links between TGFβ and Notch signaling in angiogenesis.
Methods
Animal
Mgp+/− mice (B6.129S7-Mgptm1Kry/KbosJ) on C57BL/6J background were obtained from the Jackson Laboratory. The genotypes were confirmed by PCR [28], and the experiments were performed with generation F4-F6. Littermates were used as wild type controls. All mice were fed a standard chow diet (Teklad Rodent Diet 8604, Envigo, Placentia, CA). The use of animals and all the experimental procedures were reviewed and approved by University of California Los Angeles (UCLA) Chancellor’s Animal Research Committee and conducted in accordance with the animal care guidelines set by UCLA. The investigation conformed to the National Research Council, Guide for the Care and Use of Laboratory Animals, Eighth Edition (Washington, DC: The National Academies Press, 2011).
Cell lines
Human umbilical vein endothelial cells (HUVECs) were obtained from ThermoFisher Scientific (Waltham, MA) and cultured in Medium 200 Phenol Red-Free (ThermoFisher Scientific), supplemented with Low Serum Growth Supplement (LSGS) (ThermoFisher Scientific) at 37°C in a humidified chamber with 95 % air and 5% CO2.
RNA analysis
Real-time PCR analysis was performed as previously described [29]. GAPDH was used as a control gene. Primers and probes for human BMP9, HOXD3, MGP, TGFβ1, ALK1, ALK5, Jagged1, Jagged2, Delta-like 4 (Dll4) and GAPDH were obtained from Applied Biosystems (Foster City, CA) as part of TaqMan® Gene Expression Assays.
Immunostaining
Tissue sections were fixed, processed, and stained as previously described [28]. Immunofluorescence was performed as previously described in detail [30]. Cultured cells were grown in chamber slides and fixed in 4% (weight/volume) paraformaldehyde for 30 min, permeabilized with 0.2% Triton X-100, blocked with 5% goat serum in phosphate-buffered saline (PBS), and stained using the same protocol as for tissue. We used specific antibodies for pSMAD1 (Millipore, St. Louis, MO), pSMAD2 (ThermoFisher Scientific), HOXD3 (Santa Cruz Biotechnology, Dallas, TX), TGFβ1 and ALK1 (R&D Systems, Minneapolis, MN), and ALK5 (Sigma-Aldrich, St. Louis, MO). The nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich).
Immunoblotting
Total cell lysates were obtained by lysing the cells in RIPA lysis and extraction buffer (Thermo Fisher Scientific). The protein concentration was determined using the Pierce BCA Protein Assay Kit (ThermoFisher Scientific). The proteins were separated using 12% SDS-PAGE gels (ThermoFisher Scientific) and transferred to nitrocellulose membrane, then incubated with the primary antibodies. The antibodies included phosphorylated (p)-SMAD1/5/8 (Millipore, diluted 1:500), p-SMAD2 (ThermoFisher Scientific, diluted 1:500), HOXD3 and GAPDH (Santa Cruz Biotechnology, diluted 1:200), ALK5 (Sigma-Aldrich, diluted 1:500), TGFβ1 and ALK1 (both from R&D Systems, diluted 1:500). After the membrane was washed three times with Tris-buffered saline with Tween 20 (TBST), it was incubated with secondary antibodies, such as goat anti-rabbit antibodies or goat anti-mouse antibodies (both from Cell Signal Technology, Danvers, MA, diluted 1:1000) or donkey anti-goat antibodies (R&D Systems, diluted 1:5000). The protein expression was normalized to β-actin in each sample.
Angiogenesis assay
Angiogenesis assays were performed as previous described [28]. 96-well plates were pre-coated with 50 μl of Matrigel/well (BD Bioscience, San Jose, CA), which was allowed to polymerize for 30 min at 37°C. HUVECs (4 × 104 cells/well) were resuspended in 100 μl of culture medium and seeded in the Matrigel-coated 96-well plate. HUVECs were incubated for 6 hours to allow formation of tube-like structures. The vascular coverage was measured and compared between conditions.
Chromatin Immunoprecipitation (ChIP) Assays
A commercially available ChIP assay kit (USB Corporation, Cleveland, OH) was used to perform ChIP assay, as per the manufacturer’s protocol. Immunoprecipitation was performed using the same antibodies as were used for the immunostaining. Real-time PCR was performed using the following primers for the TGFβ1 promoter: (F) 5′-GCTTCCTTGATCCCCTTCTT-3′, (R) 5′-GAACATGGATGGCAGACAAA-3′ and (F) 5′-CCCCATCTTAATCCTTGGAC-3′, (R) 5′-TAAAGCCACCACTTCCTCTCC-3′, and for HOXD3 promoter: (F) 5′-TCCACTTAGGCACTTACTGGGT-3′, (R) 5′-CAGGAACTGCTCTGGTGAACTC-3′. The conditions were as follows; 94°C denaturation, 55°C annealing, and 7200B0C extension for 45 cycles.
Euthanasia
Euthanasia is required as an endpoint in order to allow collection and analysis of tissues. Mice are killed by isoflurane followed by cervical dislocation or intraperitoneal injection of sodium pentobarbital as recommended by the Panel of Euthanasia of the American Veterinary Medical Association.
Statistical analysis
The analyses were performed using GraphPad Instat®, version 3.0 (GraphPad San Diego, CA). Data were analyzed by either unpaired 2-tailed Student’s t test or one-way ANOVA with Tukey’s multiple-comparisons test for statistical significance. Data represent mean ± SD. P-values less than 0.05 were considered significant, and experiments were repeateda minimum of three times.
Supplementary Material
Highlights.
Excess endothelial BMP activity induces Hoxd3.
BMP9/ALK1 signaling induces HOXD3 expression.
Excess BMP activity enhances TGFβ signaling mediated by HOXD3.
TGFβ1 and BMP9 stimulate the expression of MGP, which provides negative feedback and limits the enhanced ALK1 induction.
ACKNOWLEDGMENTS
Funding for this work was provided in part by NIH grants NS79353 (Y.Y.), HL139675 (Y.Y.), HL30568 (K.I.B.), and HL81397 (K.I.B.).
Abbreviations
- BMP
Bone morphogenetic protein
- TGFβ
Transforming growth factor β
- Hoxd3
Homeobox D3
- ALK1
Activin receptor-like kinase 1
- MGP
Matrix Gla protein
- Dll4
Delta-like 4
- EC
Endothelial cell
- SMAD
Mothers Against Decapentaplegic Homolog
- HUVECs
Human umbilical vein endothelial cells
- ChIP
Chromatin Immunoprecipitation
- vWF
von Willebrand factor
Footnotes
COMPETING FINANCIAL INTERESTS
The authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Condie BG, Capecchi MR. Mice homozygous for a targeted disruption of Hoxd-3 (Hox-4.1) exhibit anterior transformations of the first and second cervical vertebrae, the atlas and the axis. Development. 1993;119:579–95. [DOI] [PubMed] [Google Scholar]
- [2].Manley NR, Capecchi MR. Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev Biol. 1998;195:1–15. [DOI] [PubMed] [Google Scholar]
- [3].Boudreau N, Andrews C, Srebrow A, Ravanpay A, Cheresh DA. Induction of the angiogenic phenotype by Hox D3. J Cell Biol. 1997;139:257–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kachgal S, Mace KA, Boudreau NJ. The dual roles of homeobox genes in vascularization and wound healing. Cell Adh Migr. 2012;6:457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhong J, Eliceiri B, Stupack D, Penta K, Sakamoto G, Quertermous T, et al. Neovascularization of ischemic tissues by gene delivery of the extracellular matrix protein Del-1. J Clin Invest. 2003;112:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang L, Tong D, Guo Q, Wang X, Wu F, Li Q, et al. HOXD3 targeted by miR-203a suppresses cell metastasis and angiogenesis through VEGFR in human hepatocellular carcinoma cells. Scientific reports. 2018;8:2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Toshner M, Dunmore BJ, McKinney EF, Southwood M, Caruso P, Upton PD, et al. Transcript analysis reveals a specific HOX signature associated with positional identity of human endothelial cells. PLoS One. 2014;9:e91334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Umulis D, O’Connor MB, Blair SS. The extracellular regulation of bone morphogenetic protein signaling. Development. 2009;136:3715–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23:787–823. [DOI] [PubMed] [Google Scholar]
- [10].Scharpfenecker M, van Dinther M, Liu Z, van Bezooijen RL, Zhao Q, Pukac L, et al. BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J Cell Sci. 2007;120:964–72. [DOI] [PubMed] [Google Scholar]
- [11].Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. [DOI] [PubMed] [Google Scholar]
- [12].Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005;16:251–63. [DOI] [PubMed] [Google Scholar]
- [13].Akhurst RJ. TGF beta signaling in health and disease. Nat Genet. 2004;36:790–2. [DOI] [PubMed] [Google Scholar]
- [14].Bostrom K, Tsao D, Shen S, Wang Y, Demer LL. Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem. 2001;276:14044–52. [DOI] [PubMed] [Google Scholar]
- [15].Yao Y, Shahbazian A, Bostrom KI. Proline and gamma-carboxylated glutamate residues in matrix Gla protein are critical for binding of bone morphogenetic protein-4. Circ Res. 2008;102:1065–74. [DOI] [PubMed] [Google Scholar]
- [16].Yao Y, Jumabay M, Wang A, Bostrom KI. Matrix Gla protein deficiency causes arteriovenous malformations in mice. J Clin Invest. 2011;121:2993–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, et al. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res.107:485–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yao Y, Jumabay M, Ly A, Radparvar M, Cubberly MR, Bostrom KI. A role for the endothelium in vascular calcification. Circ Res. 2013;113:495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yao J, Guihard PJ, Blazquez-Medela AM, Guo Y, Moon JH, Jumabay M, et al. Serine Protease Activation Essential for Endothelial-Mesenchymal Transition in Vascular Calcification. Circ Res. 2015;117:758–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. [DOI] [PubMed] [Google Scholar]
- [21].Bidart M, Ricard N, Levet S, Samson M, Mallet C, David L, et al. BMP9 is produced by hepatocytes and circulates mainly in an active mature form complexed to its prodomain. Cell Mol Life Sci.69:313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K. Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem. 2006;281:33921–30. [DOI] [PubMed] [Google Scholar]
- [23].Wu X, Yao J, Wang L, Zhang D, Zhang L, Reynolds EX, et al. Crosstalk between BMP and Notch Induces Sox2 in Cerebral Endothelial Cells. Cells. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yao Y, Yao J, Radparvar M, Blazquez-Medela AM, Guihard PJ, Jumabay M, et al. Reducing Jagged 1 and 2 levels prevents cerebral arteriovenous malformations in matrix Gla protein deficiency. Proc Natl Acad Sci U S A. 2013;110:19071–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Majesky MW. Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2016;36:e82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, et al. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104:3225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Yao Y, Shao ES, Jumabay M, Shahbazian A, Ji S, Bostrom KI. High-density lipoproteins affect endothelial BMP-signaling by modulating expression of the activin-like kinase receptor 1 and 2. Arterioscler Thromb Vasc Biol. 2008;28:2266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yao Y, Jumabay M, Ly A, Radparvar M, Wang AH, Abdmaulen R, et al. Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood. 2012;119:5037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bostrom KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2011;108:446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jumabay M, Abdmaulen R, Urs S, Heydarkhan-Hagvall S, Chazenbalk GD, Jordan MC, et al. Endothelial differentiation in multipotent cells derived from mouse and human white mature adipocytes. Journal of molecular and cellular cardiology. 2012;53:790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.