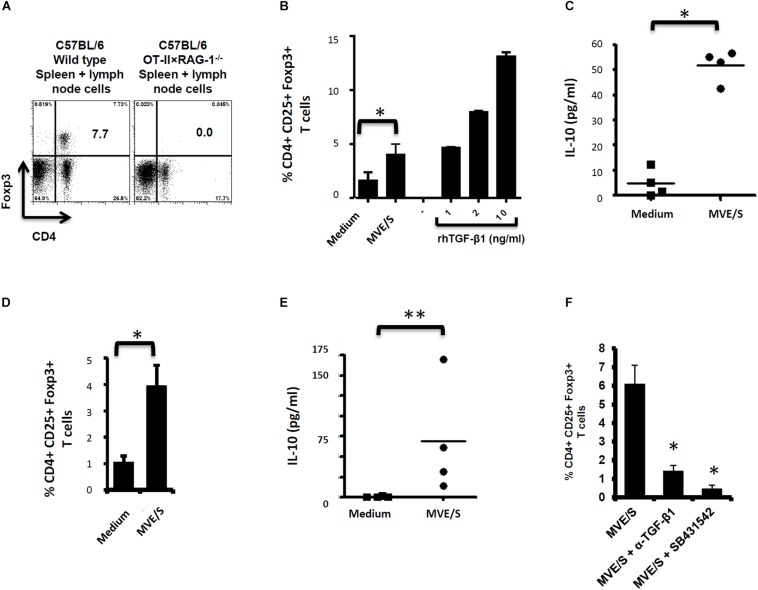
FIGURE 2.
E/S products of E. multilocularis metacestode promote the de novo Foxp3+ Treg conversion and IL-10 production by naïve T cells in vitro. (A) Staining of CD4+ Foxp3+ T-cell within the bulk of spleen and lymph node cells from wild type C57Bl/6 or C57Bl/6 OT-II.RAG-1–/– mice over a C57Bl/6 background. This shows the lack of Foxp3 + cells among CD4 + T cells when isolated from the lymphoid organs of naïve C57Bl/6 OT-II.RAG-1–/– mice. (B) MVE/S promote de novo CD4+CD25+Foxp3+ Treg conversion in vitro. Freshly generated DCs (Day 8, from 3 bone marrow cell preparations) were co-cultured individually with 3 cell preparations of naïve CD4+CD25- T-cells from 3 OT-II.RAG-1–/– mice at a DC:T-cell ratio of 1:3 in R10 medium supplemented with OVA peptide (200 ng/ml). E/S-free serum-supplemented medium (DMEM10 redox) or MVE/S-containing (DMEM10 redox) medium was added to the cultures prior to incubation. Different doses of recombinant human TGF-β1 were used as positive controls. 5 days later, cells were harvested and stained for CD4, CD25 and Foxp3 prior to flow cytometry analysis. (C) Additionally, culture supernatants were collected and probed for IL-10 by ELISA. (B,C) Summarized in the graph are the percentages of CD25+ Foxp3+ cells within the CD4+ T-cell population and the production of IL-10 measured after exposure to the indicated stimuli. Data represent mean ± SD from two independent experiments with products from two different parasite isolates. (D) Foxp3+ Treg frequencies in CD4+ T cells cultured for 5 days on CD3/CD28 antibody-coated plates in the presence of E/S-free medium (DMEM10 redox) or MVE/S-containing medium. Bars represent the mean ± SD of results obtained with E/S products from 4 different parasite isolates tested in 2 independent experiments on fresh T-cell preparations. ∗p < 0.05. (E) Naïve CD4+ CD25– T-cells freshly isolated from C57Bl/6 mice were stimulated at 2 × 105/ml with CD3/CD28 antibodies in the presence of parasite E/S-free cultivation medium (DMEM10redox) or MVE/S-containing (DMEM10 redox) medium. After 72 h, the T-cells supernatants were collected and probed for IL-10 concentration by Elisa. Horizontal bars represent the mean from experiments conducted with E/S products from 4 different parasite isolates tested in 2 independent experiments on fresh T-cell preparations. Data were compared using Mann–Whitney U test ∗p < 0.05. ∗p < 0.05; ∗∗p < 0.005. (F) Blocking TGF-β signaling or host TGF-β alone abrogates E. multilocularis-driven Treg conversion in vitro. Mean percentages of Foxp3+ Treg within the CD4+ T-cell population of OT-II naïve CD4+ T-cells cultivated with freshly generated DC (Day 8) at a DC:T-cell ratio of 1:3 in R10 medium supplemented with OVA peptide (200 ng/ml) in the presence of MVE/S-containing medium alone (supplemented with DMSO in one out of two experiments), combination of MVE/S-containing medium with TGF-β antibody or combination of MVE/S-containing medium with SB431542 (resuspended in DMSO). Flow cytometry was performed 5 days later. Bars represent mean ± SD from two independent experiments with fresh DC/T cell preparation in each experiment. Data were compared using Mann–Whitney U test ∗p < 0.05.