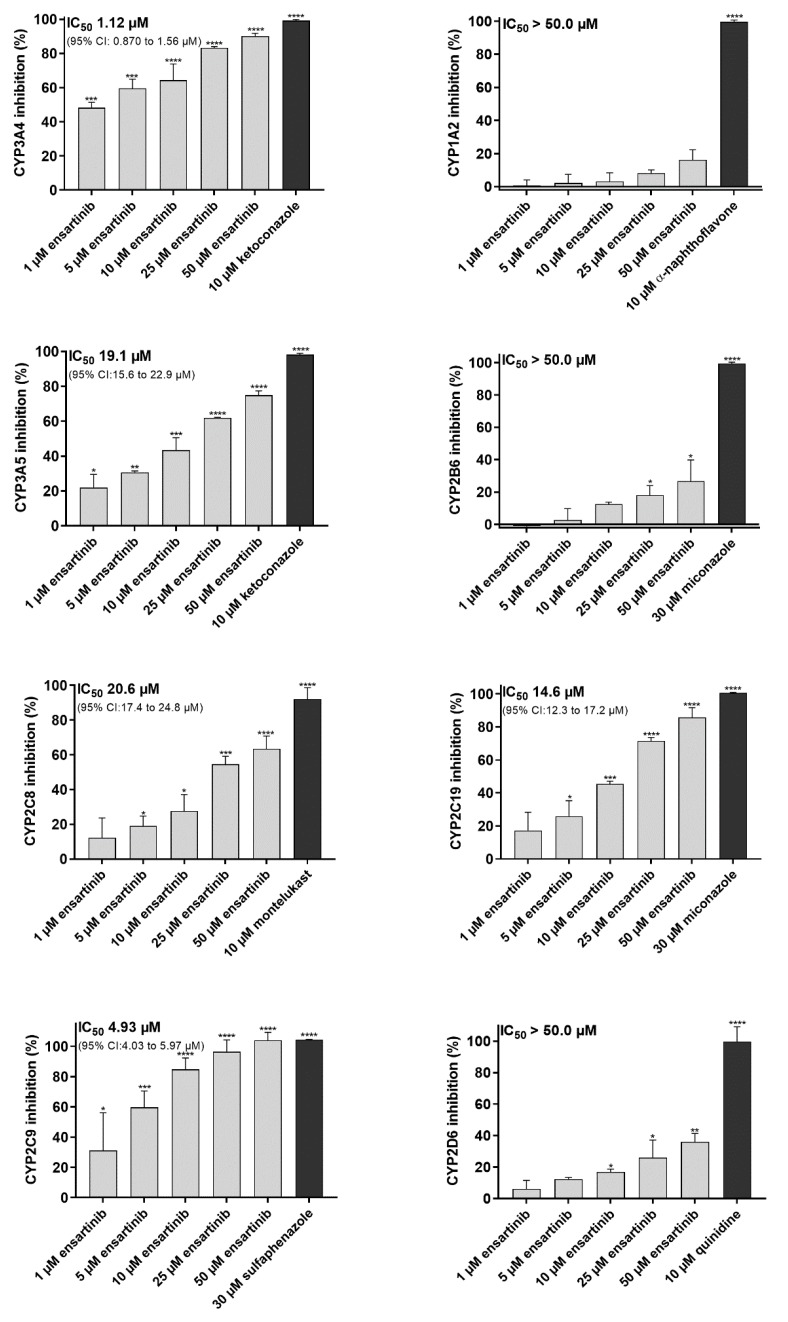
Figure 3.
Effect of ensartinib on the activities of human cytochrome P450 (CYP)1A2, CYP3A4, CYP3A5, CYP2B6, CYP2C19, CYP2C8, CYP2C9, and CYP2D6 isoforms assessed using the commercial Vivid CYP Screening Kits. Ensartinib and model inhibitors were pre-incubated with enzymes for 10 min, after which the reaction was initiated by adding a mixture of NADP+ and the appropriate Vivid substrate. Raw fluorescence measurements acquired after 15 minutes’ incubation were normalized against reference 100% and 0% activity values. Maximal (100%) enzyme activity was represented by the fluorescence of samples containing only the enzyme and 0.5% DMSO with no drug. The level of fluorescence corresponding to 0% activity was determined by analyzing samples incubated with the enzyme solvent buffer without the enzyme and 0.5% DMSO. Absolute IC50 values were determined by assuming that the model inhibitor response represented 100% inhibition. The plotted values are means ± SD based on three independent experiments. Statistical analysis was performed with background-subtracted raw fluorescence data using one-way ANOVA followed by Dunnett’s post hoc test (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 compared to 100% activity control).