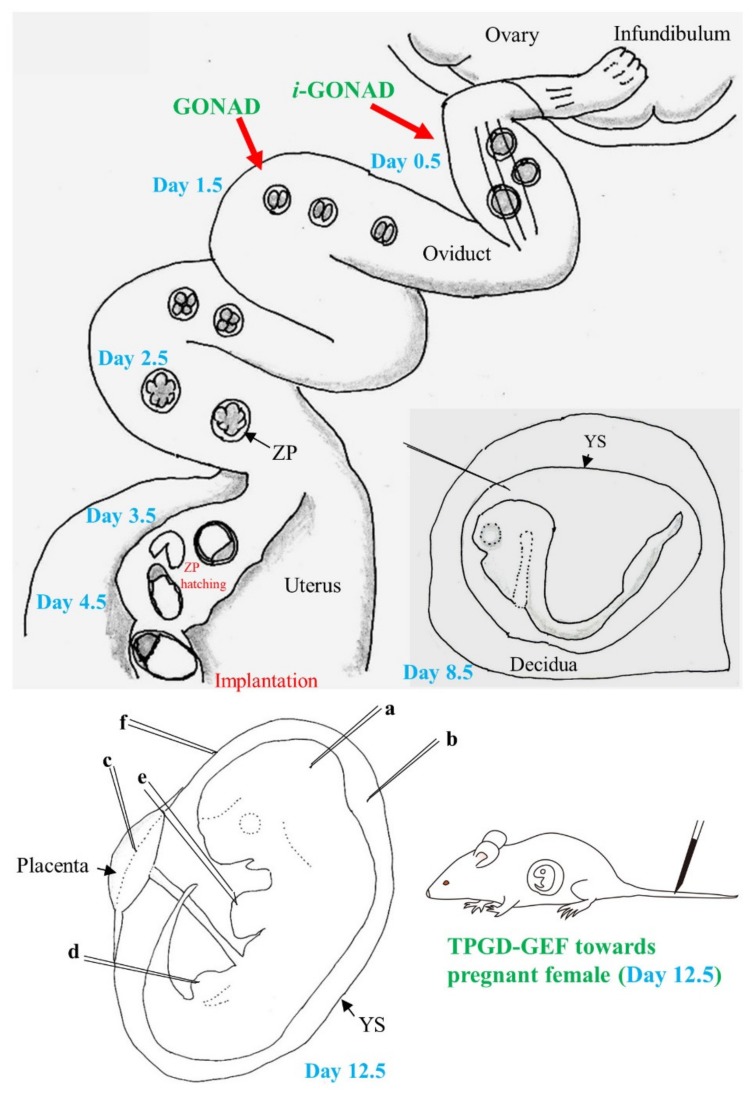
Figure 1.
Schematic representation of preimplantation (days 0.5 to 4.5; day 0 of pregnancy is defined as the day a vaginal plug is found) and postimplantation (days 5.5 to 9.5 and 11.5) development of mice: During the preimplantation stage, zygote (1-cell embryo) (at day 0.5), 2-cell embryo (at day 1.5), 8- to 16-cell embryo (at day 2.5), early blastocyst (at day 3.5), and late blastocyst (at day 4.5) float in the oviductal lumen or uterine horn. Embryos at days 0.5 to 4.5 have zona pellucida (ZP), but embryos at day 4.5 begin to escape from ZP, which is called “ZP hatching”, and form early egg cylinder containing epiblast after implantation. In vivo genome editing is possible after intraoviductal instillation of a solution containing the genome-editing components and subsequent in vivo electroporation (EP), known as the “genome-editing via oviductal nucleic acids delivery (GONAD)” (for 2-cell embryos) and “improved GONAD (i-GONAD)” (for zygotes) techniques. On day 8.5, embryos commence somite formation with the formation of blood-island and a beating heart. Furthermore, closure of neural tube commences. Notably, embryos at days 5 to 8 are surrounded by decidua, which does not allow visualization of embryos upon surgical dissection of the uterus. Transfection of day 8.5 embryos is possible when a nucleic acid-containing solution is injected into the internal portion of decidua through the yolk sac (YS) using a micropipette. On day 12.5, the fetus (embryo) is visible through the YS upon surgical dissection of the uterus under a dissecting microscope. Thus, it is possible to administer intrabrain (a), intraamniotic (b), intraplacental (c), intramuscular (d), intracardiac (e) and intravitelline (f) injections using a micropipette for in utero gene delivery. Tail-vein injection of a solution containing genome-editing components into pregnant female mice is also a useful in vivo approach to induce genome editing in day 12.5 fetuses, which is called “transplacental gene delivery for acquiring genome-edited fetuses (TPGD-GEF)”.