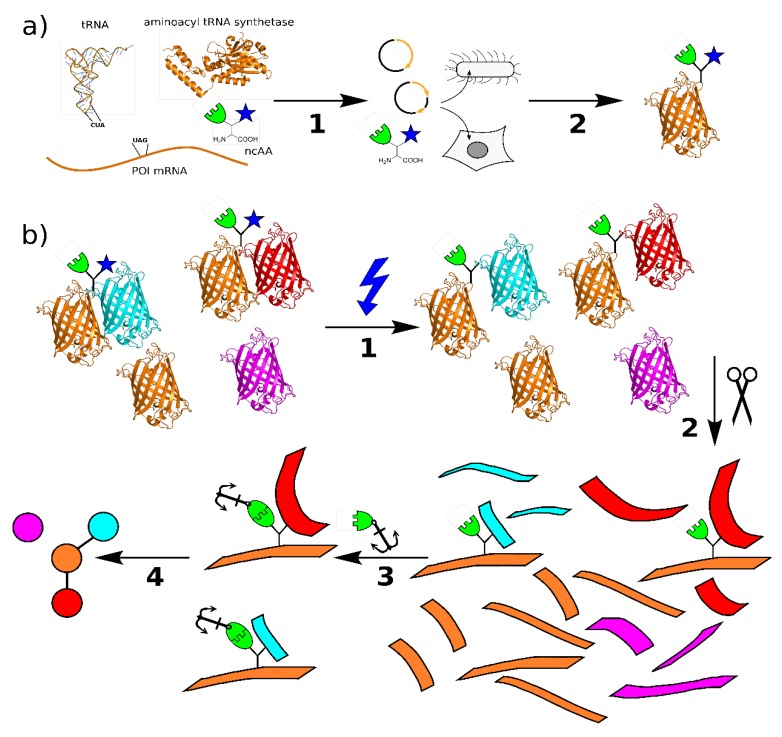
Figure 1.
(a) Principle of genetic code expansion: The four components required are the mRNA for the target protein with a stop codon at the intended incorporation site, a tRNA complementary to this codon, the non-canonical amino acid, and an aminoacyl-tRNA synthetase that recognizes and connects the tRNA complementary to the TAG stop codon (tRNACUA) and non-canonical amino acid (ncAA). In step 1, these components are introduced into the host organism, e.g., by transient transfection, which then produces the protein of interest (POI) with the ncAA at the selected site in step 2. (b) Principle of protein interaction analysis using bifunctional amino acids: The POI incorporating the bifunctional ncAA binds to its native interaction partners in vitro or in vivo. Upon irradiation with UV light (1), a covalent bond is formed between the photo-crosslinker (blue) and the binding partner. The sample is processed by protease digestion (2) and the peptides containing the ncAA are labeled via the click chemistry handle (3). The relevant peptides are enriched by pulldown and analyzed by mass spectrometry. From these data. the protein interactions can be mapped (4) (structures: PDB 1EHZ, 6AAC, and 1GFL).