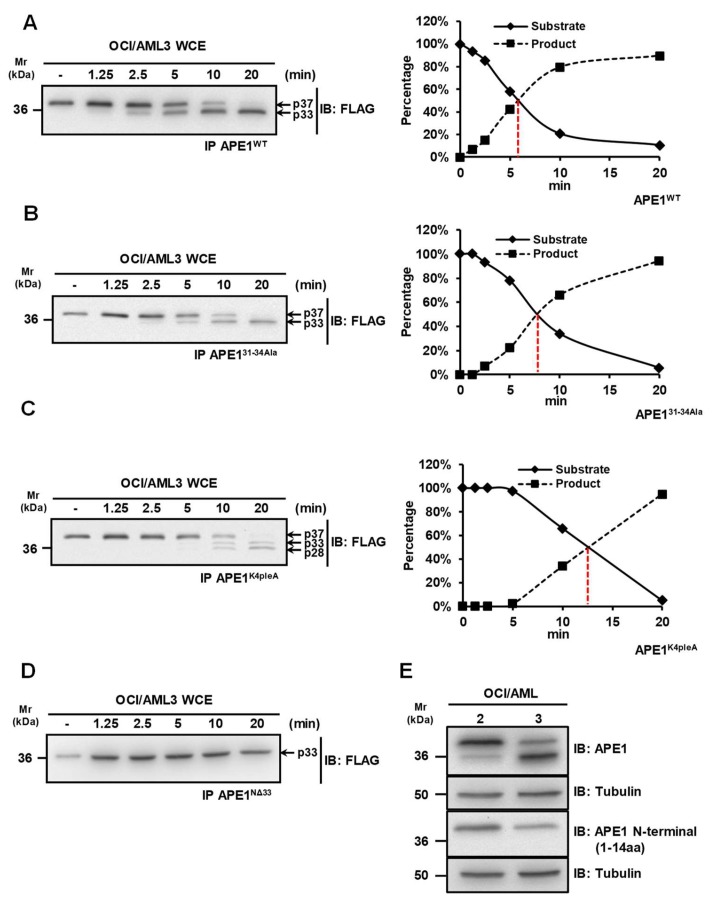
Figure 1.
A protease activity is responsible for the cleavage of the APE1 N-terminal domain. (A–D) Kinetic measurements for the protease activity towards different APE1 mutants. Whole OCI/AML3 cell extracts (10 µg) were incubated for the indicated times, at 37 °C, with immunopurified FLAG-tagged APE1 recombinant proteins expressed in HeLa cells in the wild-type form (APE1WT) (A), the APE131-34Ala form where residues 31-34 were mutated to Ala (B), the APE1K4pleA form where Lys27, Lys31, Lys32 and Lys35 were mutated to Ala (C), and the APE1NΔ33 form lacking 33 amino acids at protein N-terminus (D). Reactions were separated onto 12% SDS-PAGE, immunoblotted and analyzed using an antibody specific for the FLAG-tag. A representative Western blotting analysis shows the cleavage of APE1 at different times. In the diagrams on the right, the quantification of the densitometric signals of the non-cleaved form of APE1 (S) and the truncated form (P) is reported. (E) The N-terminal domain of APE1 is truncated in OCI/AML3 cells. Representative Western blotting analysis on OCI/AML 2 and 3 whole cell extracts (10 µg), showing the N-terminal truncation of APE1 in OCI/AML3 cells. Antibodies used are indicated on the right-hand side. Tubulin was used as loading control.