Figure 5.
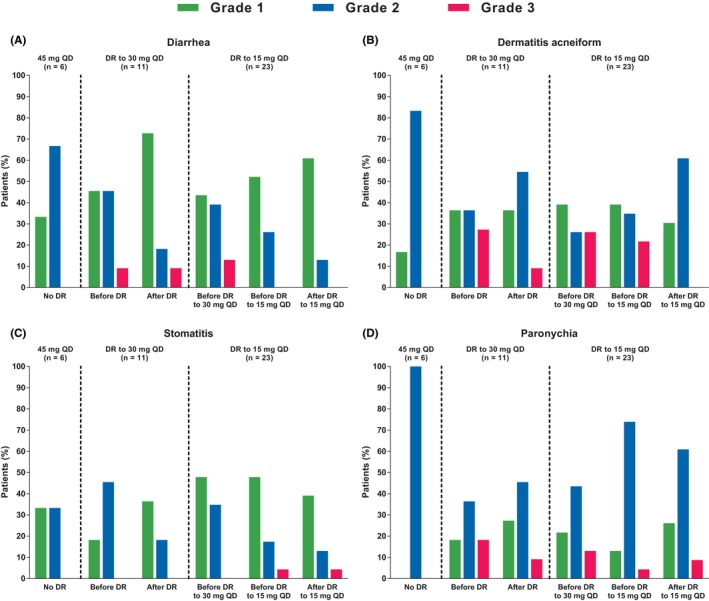
Incidences and severity of adverse events of interest before and after dacomitinib dose reduction (Japanese safety population; cutoff date: July 29, 2016). (A, diarrhea; B, dermatitis acneiform; C, stomatitis; D, paronychia. QD, once daily). The incidences and severity of the adverse events are summarized in patients who did or did not undergo dose reductions because of adverse events. The frequencies of adverse events in the interval before the dose reductions and in the interval after dose reductions are indicated. For diarrhea, dermatitis acneiform, stomatitis, or paronychia, there were no Grade 4 adverse events requiring dose reductions
