Figure 6.
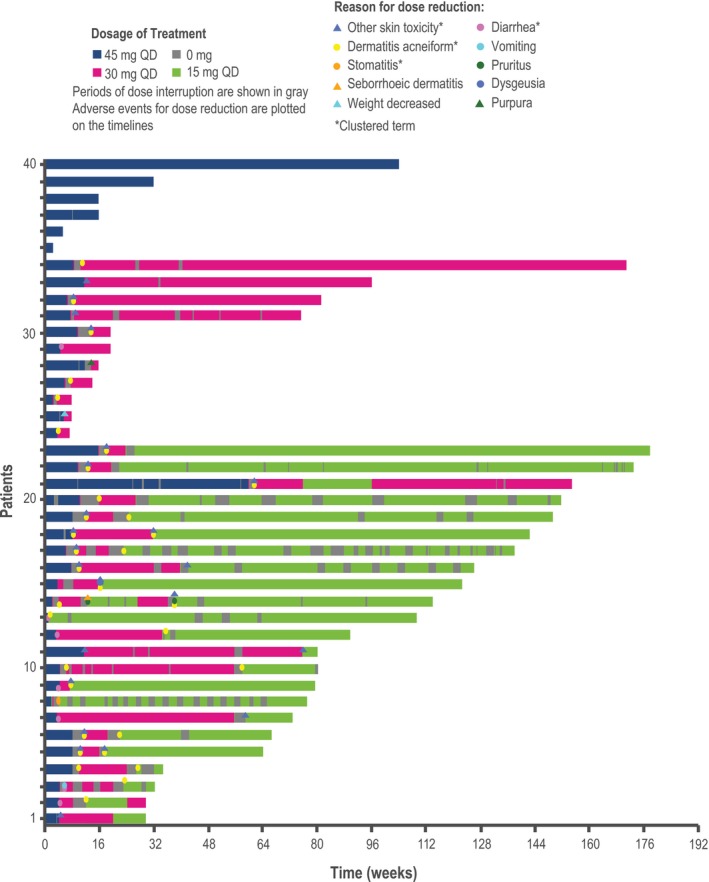
Treatment duration and dacomitinib dose adjustments (Japanese safety population; cutoff date: February 17, 2017). QD, once daily. The duration of treatment with 45 mg QD is shown in blue, with 30 mg QD in red, and with 15 mg QD in green. The period of dose interruption is shown in gray. Adverse events leading to dose reduction are plotted on the timing. The other skin toxicity cluster term includes dry skin, nail disorder, palmar‐plantar erythrodysesthesia syndrome, paronychia, skin fissures, skin ulcer, or xerosis; the diarrhea cluster term includes acute prerenal failure, azotemia, dehydration, diarrhea, blood urea nitrogen/creatinine ratio increased, electrolyte imbalance, hypovolemia, or prerenal failure; the dermatitis acneiform cluster term includes any preferred term within the high level term acnes, drug eruption, rash, rash erythematous, rash generalized, rash maculopapular, or rash pruritic; the stomatitis cluster term includes any preferred term within the high level term stomatitis and ulceration, cheilitis, oral pain, or oropharyngeal discomfort, oropharyngeal pain or mucosal inflammation
