Figure 3.
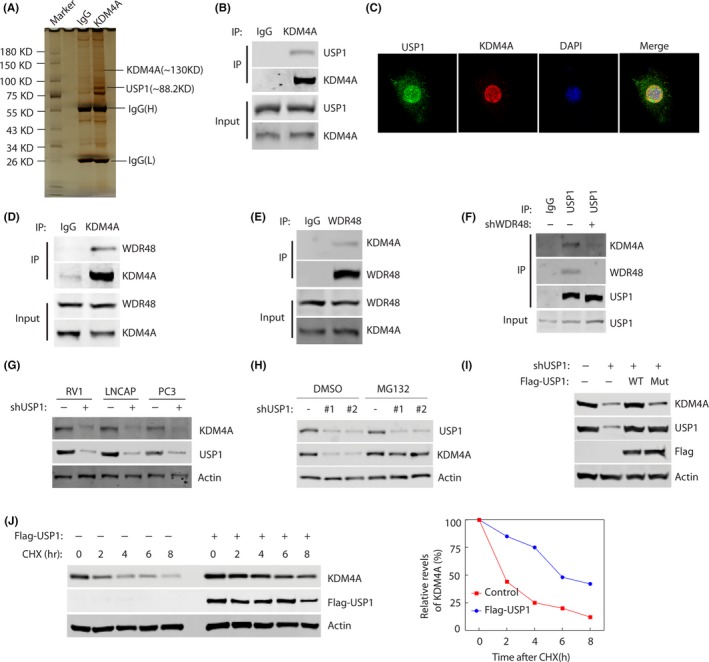
USP1 binds and stabilizes KDM4A. A, Proteins that interacted with KDM4A were identified by Co‐IP and mass spectrometry assays. B, RV1 cells were transfected with Flag‐KDM4A plasmids for 48 h, and lysates were subjected to immunoprecipitation with anti‐KDM4A Ab. Bound proteins were analyzed by western blotting with anti‐KDM4A or USP1 Abs. C, Representative fluorescence images of KDM4A and USP1 proteins in RV1 cells. Cells were seeded on coverslips for 24 h and then stained for KDM4A (red) and USP1 (green). Nuclei were visualized by DAPI staining (blue). D, E, RV1 cells were lysed and immunoprecipitated (IP) with indicated Abs. Immunocomplexes were subjected to western blotting. F, RV1 cells stably expressing control or WDR48 shRNAs were lysed and IPed with indicated Abs. Immunocomplexes were subjected to western blotting. G, RV1 cells stably expressing control or USP1 shRNAs were subjected to western blotting to examine the indicated proteins. H, Cells were untreated or treated with 10 μmol/L MG‐132 and western blotting was carried out to examine the indicated protein levels. I, 293T cells were transfected with indicated constructs. Indicated proteins were analyzed by western blotting. J, RV1 cells were stably expressed control or Flag‐USP1 constructs and then cycloheximide (CHX) pulse‐chase assay was undertaken in cells. Right panel, protein levels of USP1 relative to β‐actin
