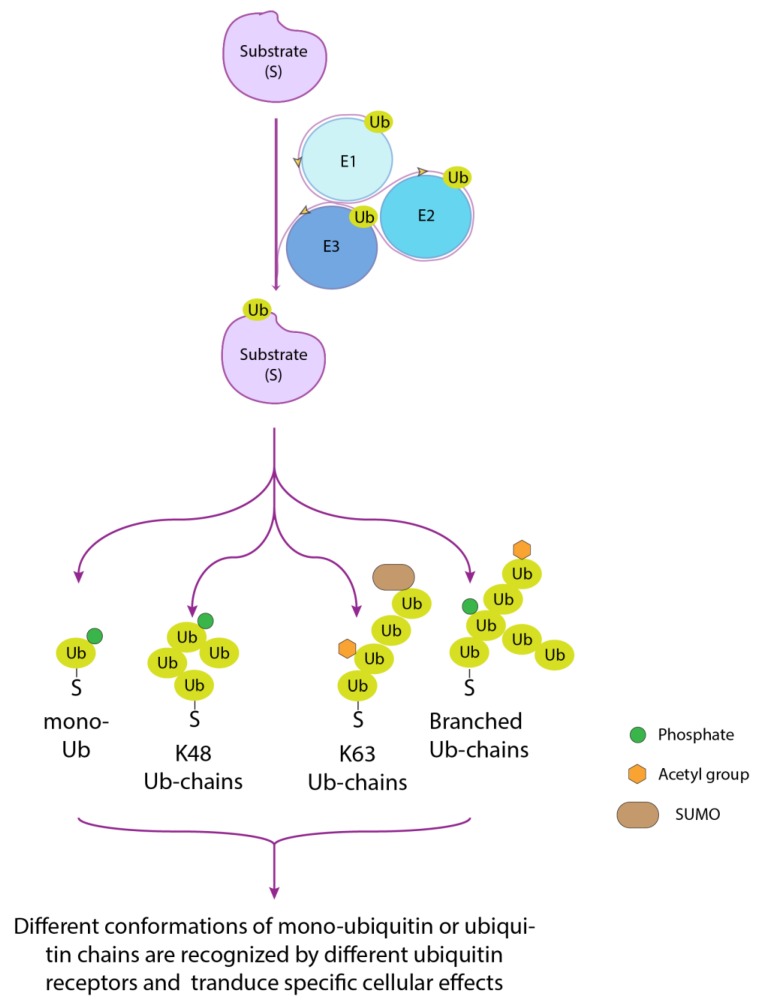
Figure 1.
An enzymatic cascade involving an activating enzyme (E1), a conjugating enzyme (E2) and a ligase (E3) carries out protein substrate ubiquitination. The ubiquitin moiety conjugated to a substrate can be targeted for additional ubiquitination cycles, potentially involving all seven ubiquitin lysines (K6, K11, K27, K29, K33, K48 and K63) and the first methionine (M1). Different chain topologies are generated according to the specific branching. The conjugated ubiquitin moieties can be further modified by different post-translational modifications, such as phosphorylation, acetylation and sumoylation. Different polyubiquitin chains, as well as the presence of other post-translational modifications, determine differences in the conformations of the protein chains and therefore have specific effects on the protein to which they are attached. The association of protein post-translational modifications (PTMs) shown on the diagram is to be considered only as an example.